Signalment:
Gross Description:
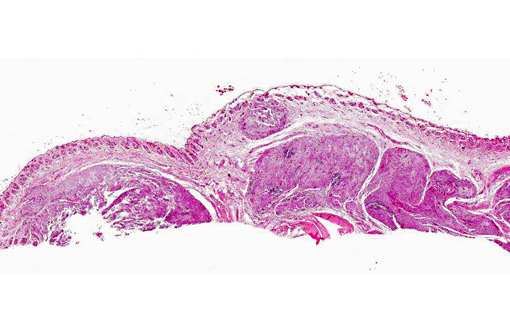
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
S. schenckii belongs to the kingdom Fungi and is a eukaryotic organism that has no mobility, is heterotrophic, and presents chitin on its cell wall. For several years, this fungus was included in division Eumycota, subdivision Deuteromycotina, class Hyphomycetes, order Moniliales, and family Moniliaceae. After a substantial fungal taxonomy revision, this fungus was replaced in division Ascomycota, class Pyrenomycetes, order Ophiostomatales, and family Ophiostomataceae.
S. schenckii must not be considered a single species. A recent molecular study demonstrated that S. schenckii is a complex of cryptic species, denominated the S. schenckii complex. Within this complex, at least five species are considered of interest due to their pathogenic potential. Gene sequencing has revealed the following six species in the complex: S. albicans, S. brasiliensis, S. globosa, S. luriei, S. mexicana and S. schenckii. These cryptic species were further subdivided into a number of smaller groups that appear to be reproductively isolated in nature. This suggests not only that the existing S. schenckii populations are in the process of divergence but also that all of the resulting lineages are undergoing separation into distinct taxa. In Brazil there are four species recognized as pathogens for human and animals: S. albicans, S. brasiliensis, S. luriei, and S. schenckii.
Although the genetic separation is considerable among the three major monophyletic clades (i.e., the Spanish clade, the Brazilian clade, and the clade made up of the rest of the South American isolates), each of them shows a high level of clonality. Primitive populations were probably isolated by the separation of the continents, and the formation of natural barriers facilitated their speciation as they became adapted to hosts endemic to the different regions. However, although geographical separation of the main clades is clearly evident, the different genotypes present within them are not related to geography, which seems to indicate that there has been interbreeding within these isolated populations.
In the environment, at temperatures ranging from 25 to 30°C, S. schenkii grows as filamentous mold; it forms white to cream-colored colonies which become brown to black in weeks and produces dark or hyaline conidia arranged along hyphae with a bouquet-like appearance. In the wild, the fungus is a saprophyte on living and decaying vegetation. Soil is fundamental for mycelium development. At 37°C, S. schenkii grows as yeast-like cell.
S. schenckii virulence factors are thermotolerance and the ability to synthesize melanin that enhances resistance to macrophage phagocytosis, allowing the first steps of infections in mammalian hosts; melanization also promotes the formation of multifocal granuloma. Another virulence factor is expression of integrins or adhesion lectin-like molecules that recognize fibronectin from the host. The fibronectin adhesins are located on the surface of yeast cell and the existence of these adhesins would favor adherence to host tissues and fungal dissemination. Components of the S. schenckii cell wall that act as adhesins and immunogenic inducers, such as a 70-kDa glycoprotein, are apparently specific to this fungus and they seem to participate in adhesion to the dermal extracellular matrix. The main glycan peptido-rhamnomannan cell wall component is the only O-linked glycan structure known in S. schenckii, and it causes depression of immune response until the sixth week of infection. Yeast cells also synthesize ergosterol peroxide that is a protective mechanism to evade reactive oxygen species during phagocytosis and may represent a virulence factor.
Differences in virulence between clinical and environmental strains were reported, but no correlation was found with the different clinical forms of sporotrichosis. In several in vitro antifungal susceptibility studies of clinical isolates of S. schenckii, a wide range of susceptibility to different drugs has been demonstrated. This suggests that these isolates could represent different species. If true, knowledge of their various responses to antifungal agents would be critical for appropriate patient management.
Classically, the infection is acquired through traumatic implantation of S. schenckii present in organic matter. The most frequent clinical presentations are the cutaneous and subcutaneous forms with or without regional lymphatic involvement. But, inhalation of the conidia can lead to pulmonary infection which, rarely, may also spread to bones, eyes, central nervous system and viscera. Another mode of transmission is through animal bites and scratches.
Sporotrichosis usually occurs in isolated cases or in small family or professional outbreaks. Epidemics are rare and, when they occur, are commonly related to a single source of infection. Interhuman transmission is rare and human sporotrichosis has sporadically been related to the scratch or bite of infected animals. The role of felids in the transmission of the mycosis to humans has, however, gained importance. There was no description of epizootics before a cat-transmitted epidemic was reported in Rio de Janeiro, Brazil, in 1980. Since then, successive reports of epizootics from different geographical regions have characterized a new risk group for acquisition of sporotrichosis, composed of cat owners and veterinarians.
It should be noted that the highest number of cases that occurred during Rio de Janeiro epizootics were in an area characterized by underprivileged socioeconomic conditions and precarious health services. The typical human patients were female, mainly housewives, which is normal if we consider that members of this group are those most frequently exposed to the fungus because they care for cats. Some authors explained the wide dissemination of the disease with factors related to the behavior of cats which, although cohabiting with human beings, do not always stay in the house but also circulate in the neighborhood, often getting involved in fights with other animals and coming into contact with soil and plants.
It is possible that environmental factors, increased urbanization, and improved diagnostics partly explain the alterations in the profile of the disease. Furthermore, since sporotrichosis is not a reportable disease in most countries, such as Brazil, there is little information on the incidence, and the known data are those generated by case publications.
Following inoculation, the fungus penetrates into deeper layers of tissue where it converts into the yeast like form (37°C). It can remain in the dermis and subcutaneous tissue at the inoculation site, spread up to regional lymphatics and produce lymphangitis and lymphadenitis, or disseminate systemically through blood vessels. Furthermore, in cats, the high frequency of respiratory signs and pulmonary and nasal mucosal lesions, in addition to the isolation of S. schenckii from bronchoalveolar lavage and from the lungs of necropsied animals, suggests the epidemiologic importance of the inhalation route in the infection. Multiple skin lesions can occur because of self-trauma, grooming, and hematogenous dissemination from the lungs or perhaps from the initial skin lesion.
The lesions are characterized by a wide variety of morphologies: nodules, tubercles, pustules, cysts, gummy lesions, ulcers, vegetative lesions, and plaques, accompanied or not by lymphangitis.
Virulence is one of the factors thought to play a role in the development of sporotrichosis, but there are discordant results concerning disease evolution in experimental sporotrichosis with S. schenckii. Clinical isolates from cutaneous and disseminated infection indicate that host immune responses also substantially participate in the progress of sporotrichosis. The immunological mechanisms involved in prevention and control of S. schenckii infections are still not very well understood. However, they probably include both humoral and cellular responses, which appear to be triggered by distinct antigens. Surface cell antigens, especially some lipids, inhibit the phagocytosis process; while the humoral response is induced by secreted fungal proteins, the exoantigens are not involved in the cellular response. The innate immune response also plays a role in the pathogenesis of sporotrichosis.
The classification of clinical presentations used for humans includes several forms: lymphocutaneous, fixed cutaneous, mucocutaneous, extracutaneous, and disseminated. These categories are difficult to transpose to sporotrichosis in dogs and cats because they frequently have more than one of these forms simultaneously. Although the cutaneous lymphatic form is the most frequently seen clinical presentation in humans, this is not the case with cats and dogs. The most common lesions in dogs and cats are skin nodules and ulcers, with frequent mucosal involvement. The initial lesions are firm subcutaneous nodules that slowly soften, generally draining purulent or seropurulent content, and progress to form exudative ulcers with slightly elevated well-defined rims. In addition, dogs and cats may have extracutaneous, mainly respiratory signs, such as sneezing, nasal discharge, and dyspnea, followed by lymphadenomegaly. Other clinical signs that may be observed are anorexia, vomiting, weight loss, cough, fever, and dehydration.
According to the location of the lesions, sporotrichosis can be classified into three forms in animals. The primary cutaneous form consists of multiple scattered raised alopecic, ulcerated, crusted nodules or plaques that remain confined to the point(s) of entry of the organism. It is thought that this form results from a high degree of host immunity, preventing spread of infection. Nodules may become ulcerated and associated with seropurulent exudate and crust formation. The normal grooming behavior of cats may result in autoinoculation and spread of lesions to distant sites. The cutaneous form may have a very chronic course. An unusual case of sporotrichosis in a dog consisted of otitis externa characterized by multiple cutaneous nodules which persisted for more than five years.
The cutaneous-lymphatic form involves the skin, subcutaneous tissue, and associated lymphatics. Lesions begin as firm round nodules at the site of entry, usually on an extremity, and spread proximally along lymphatics. Lymphatic vessels become thick and corded and a series of secondary nodules forms as the infection progresses. The nodules may break open and discharge seropurulent material. Lesions may cavitate and expose extensive areas of underlying muscle and bone. Regional lymphadenopathy is common. This is the most common form in horses. Lesions generally involve the proximal forelimbs, chest, and thigh but usually no regional lymph node involvement is evident. Dogs usually have the cutaneous or cutaneous-lymphatic form. The head, pinnae, and trunk are involved most frequently. In cats, lesions are usually located on the head, distal limbs, and base of the tail. The initial draining puncture wounds may be indistinguishable from cat-inflicted fight wound infections.
The extracutaneous/disseminated form may involve a single extracutaneous tissue, such as osteoarticular sporotrichosis, or multiple internal organs. It develops as a sequela to cutaneous lymphatic infection or following inhalation of the fungus. The disseminated form of sporotrichosis occurs most frequently in cats, and no immunosuppressive factors are usually identified. In experimentally induced sporotrichosis in cats, organisms were shown by culture to have disseminated to viscera in 50% of the cases. Cats with disseminated sporotrichosis are often febrile, depressed, and anorexic.
In cats, as the case described, unlike humans, the low frequency of typical formed granulomas and the richness of fungal elements found in the histopathology of the skin demonstrate the increased susceptibility of animals to S. schenckii. Some investigators believe that the severity of feline sporotrichosis can be related to immunosupression caused by co-infection with feline immunodeficiency virus (FIV) or feline leukemia virus (FeLV), although reports of co-infection with FIV/FeLV and S. schenckii are very rare which renders this hypothesis unlikely.
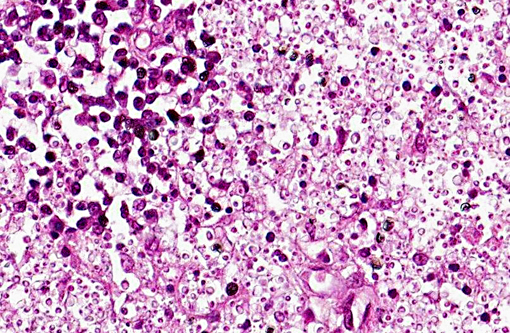
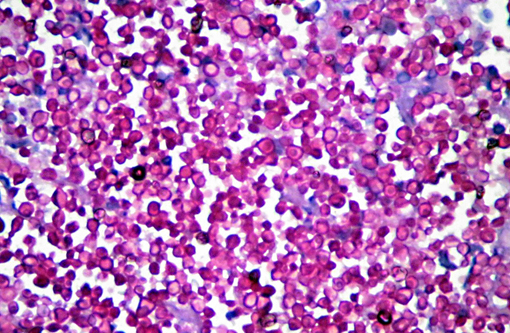
Microscopically, sporotrichosis is usually a nodular to diffuse pyogranulomatous or granulomatous inflammatory reaction involving the dermis and subcutaneous fat. The epidermis is acanthotic or ulcerated. Neutrophils, epithelioid macrophages, multinucleated giant cells, and fewer lymphocytes and plasma cells can form discrete granulomas or extensive sheets of inflammation replacing dermal and subcutaneous tissues. Fibrosis is variable, and necrosis may be extensive. Yeast(s) surrounded by a stellate radial corona of brightly eosinophilic material (asteroid body/Splendore-Hoeppli reaction) are seen in some cases. The yeasts appear as round, oval, or elongated ("cigar"-shaped) single or budding cells which measure 2-6 μm or more in diameter for the round and oval forms.
Although S. schenckii may be seen in tissue with the routinely used hematoxylin and eosin (H&E) stain, other special stains such as Gomori methenamine silver (GMS) or periodic acid-Schiff (PAS) can be employed to enhance fungal detection. Atypical S. schenckii cells can appear spherical and surrounded by a PAS-positive capsule, resembling Cryptococcus cells.
Isolation of the fungus from the nails and oral cavity of cats supports evidence indicating that transmission can occur through a scratch or bite, while isolation from the nasal fossae and cutaneous lesions indicates the possibility of infection through secretions. Sporotrichosis can be diagnosed through a correlation of clinical, epidemiological, and laboratory data. Laboratory analysis for the determination of sporotrichosis includes direct examination of specimens such as tissue biopsy specimens or pus from lesions. In case of disseminated infections, other specimens, such as sputum, urine, blood, and cerebrospinal and synovial fluids can be analyzed, depending on the affected organs.
The differential diagnoses should be considered in accordance with the diversity of clinical forms and the morphology of the lesions. The main differential diagnosis is cutaneous leishmaniasis, cryptococcosis, mycobacterioses and skin neoplasias. Sporotrichosis can also mimic cutaneous bacterial infections, sarcoidosis, lupus vulgaris, tuberculosis, and scrofuloderma, among others in humans. These conditions should be differentiated by history, areas of endemicity, and lab tests.
Sodium iodide (NaI) or potassium iodides (KI) were considered the drugs of choice in human and canine sporotrichosis; however, serious adverse effects have limited their use. Itraconazole (ITZ) is considered the drug of choice in feline and human sporotrichosis treatment because of its greater effectiveness and safety when compared to other antifungal agents. Sporotrichosis in cats is more difficult to treat than in dogs, requiring a prolonged period of therapy. The number of affected noncontiguous anatomic regions, the general medical condition, and the degree of compromise to the immune system influence the treatment outcome. The cooperation and persistence of the owners is instrumental in attempting a successful response to therapy. When sporotrichosis is not treated for an adequate time, it often recurs, usually with respiratory signs. In these cases the clinical cure is difficult; respiratory signs are associated with treatment failure and death. There is a report of ITZ-resistant strains of S. schenckii.
People handling cats with potential sporotrichosis should follow biosecurity measures. In addition, separation of sick cats from other animals in the same environment is indicated. Care must be taken to avoid cuts or penetrating injuries when working with infected cats, and protective outerwear should be worn. Proper physical restraint or sedation of noncooperative patients must be done to allow complete examination of lesions and the collection of biologic material for laboratory analysis.
Decontamination and cleaning of cages or transport containers must be done with hypochlorite (1%), diluted 1:3 in water, for at least 10 minutes. If possible, sun drying is also beneficial. Examination tables should be cleaned after contact with infected animals and disinfected with sodium hypochlorite solution (1%), followed by alcohol 70% for at least 10 minutes using disposable paper towels. Additionally, floors and walls must be cleaned and disinfected daily with sodium hypochlorite solution (1%). For public health purposes and to control epidemic cat-transmitted sporotrichosis, an effective and viable therapeutic regimen applied to cats under field conditions is necessary.
Moreover, public awareness programs on sporotrichosis prophylaxis are required, encouraging the following: responsible ownership, castration, cremation of dead cats, confinement of cats inside the home, limitation of the number of cats per household, regular cleaning of the dwelling, and proper health care for the animals.
This case is interesting because of the possibility of dealing with an ITZ resistant strain. Even if other possibilities (like inadequate treatment) cannot be excluded, such cases deserve special attention because of the discussed antropozoonotic potential and epidemic situation in some cities.
JPC Diagnosis:
Conference Comment:
References:
2. Carlos IZ, Sassa MF, Sgarbi DBG, Placeres MCP, Maia DCG. Current research on the immune response to experimental sporotrichosis. Mycopathologia. 2009;168:1-10.
3. Ginn PE. Mansell JEKL, Rakich PM. Skin appendages: Fungal diseases of skin. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed. Vol 1. New York, NY: Elsevier Saunders; 2007:694 -708.
4. Lopes-Bezerra LM. Sporothrix schenckii cell call peptidorhamnomannans. Frontiers in Microbiology. 2011;2(243):1-4.
5. Marimon R, Gene J, Cano J, trilles L, Lazera MS, Guarro J. Molecular Phylogeny of Sporothrix schenckii. J Clin Microbiol. 2006;44(9):32513256.
6. Marimon R, Cano J, Gene J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana,Three New Sporothrix Species of Clinical Interest. J Clin Microbiol. 2007;45(10):31983206.
7. Oliveira DC, Lopes PGM, Spader TB, Mahl CD, Tronco-Alves GR, Lara VM, et al. Antifungal Susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii Complex Identified in Brazil. J Clin Microbiol. 2011;49(8):30473049.
8. Schubach AO, Barros MB, Wanke B. Epidemic sporotrichosis. Current Opin Infectious Dis. 2008;21(2):129-133.
9. Schubach TMP, Menezes RC, Wanke B. Sporotrichosis. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 4th ed. Philadelphia, PA: Saunders; 2011:645-650.