WSC 22-23
Conference 1
CASE IV:
Signalment:
6-month-old, Aberdeen-Angus bull calf (Bos taurus or domesticus)
History:
The clinical signs prior to death included diarrhea, snotty nose, and a low body temperature (99-100 F). The calf had been vaccinated 2 weeks prior with a vaccine containing a modified-live Bovine Viral Diarrhea Virus (BVDV).
Gross Pathology:
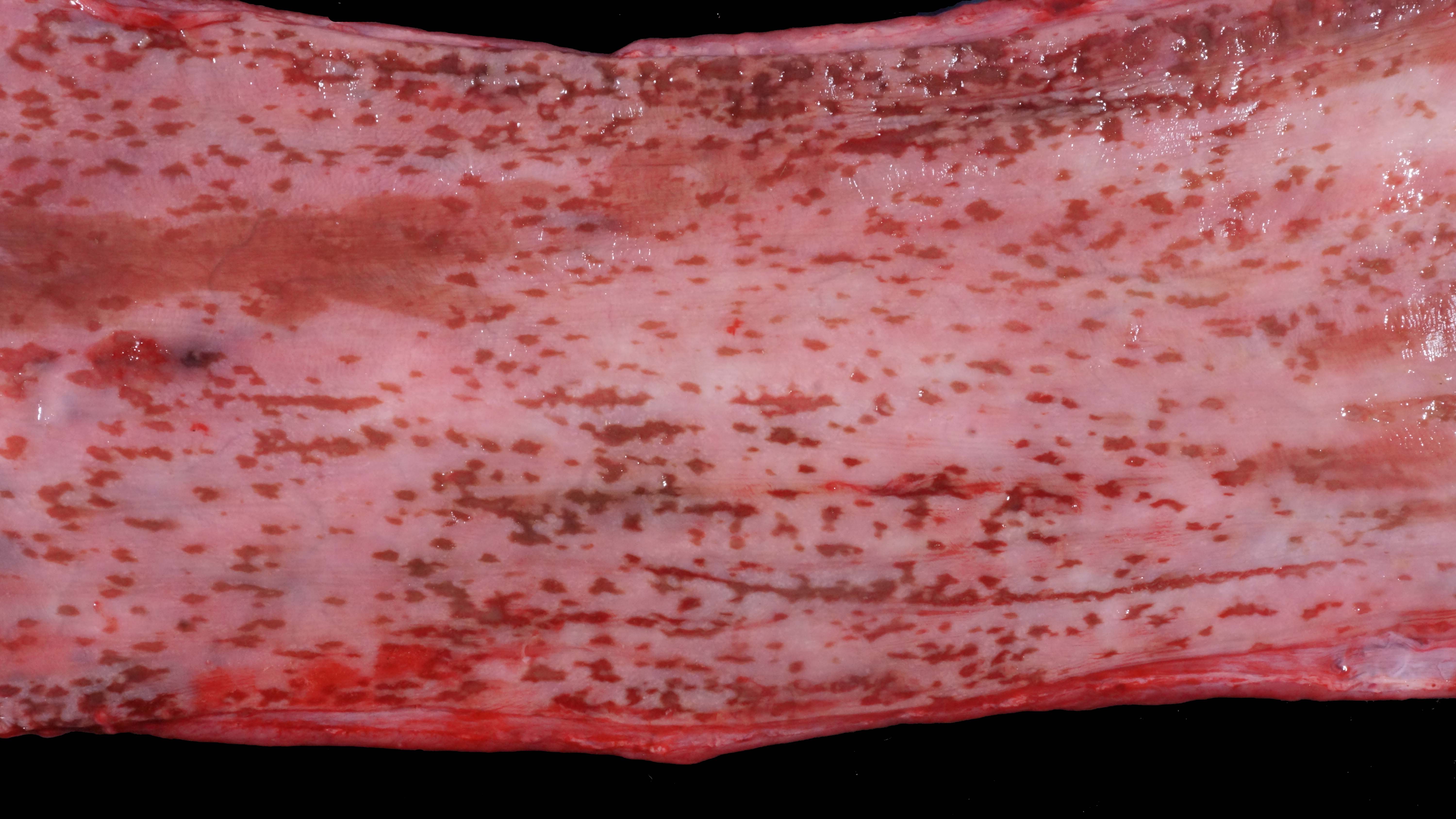
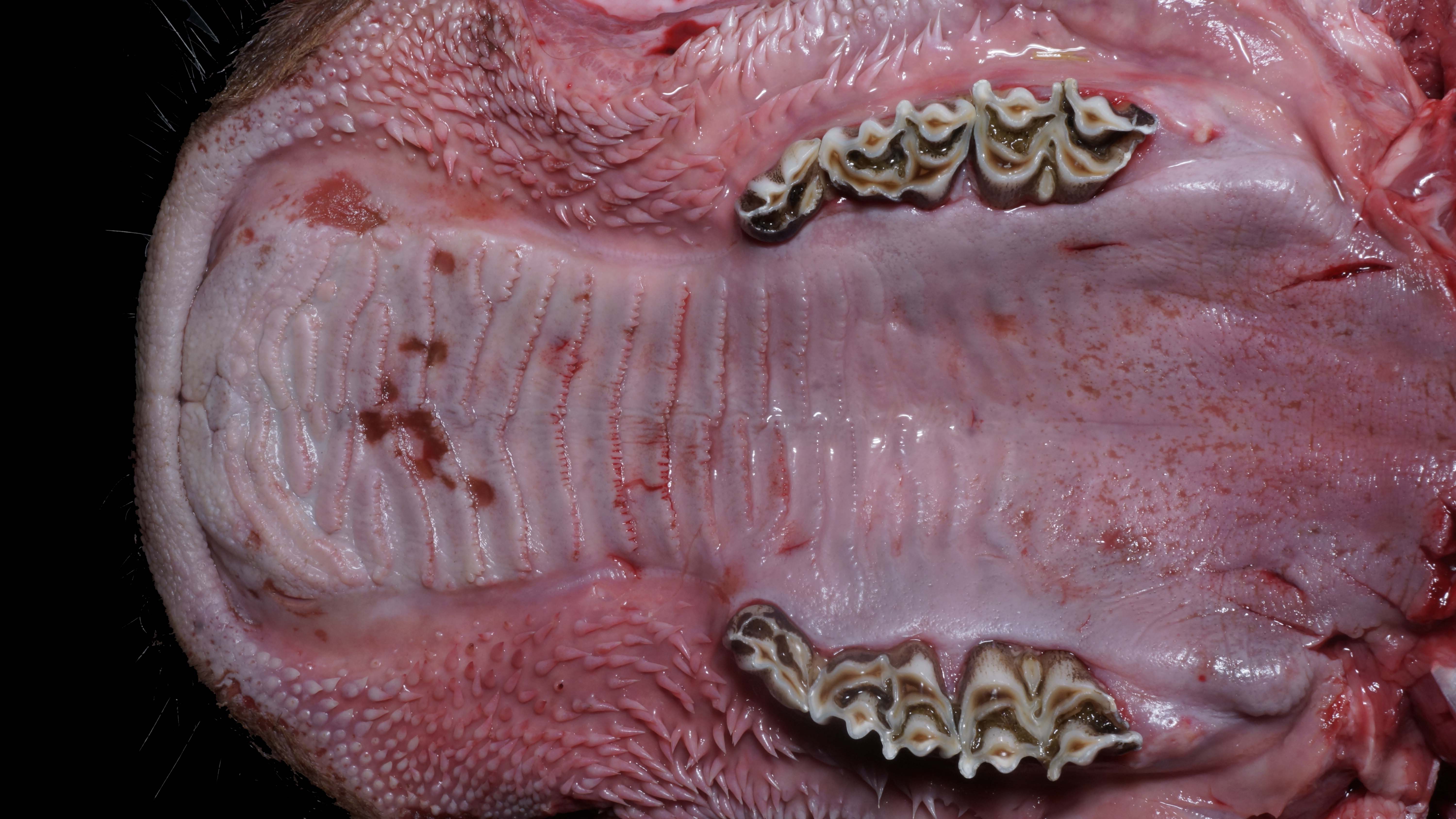
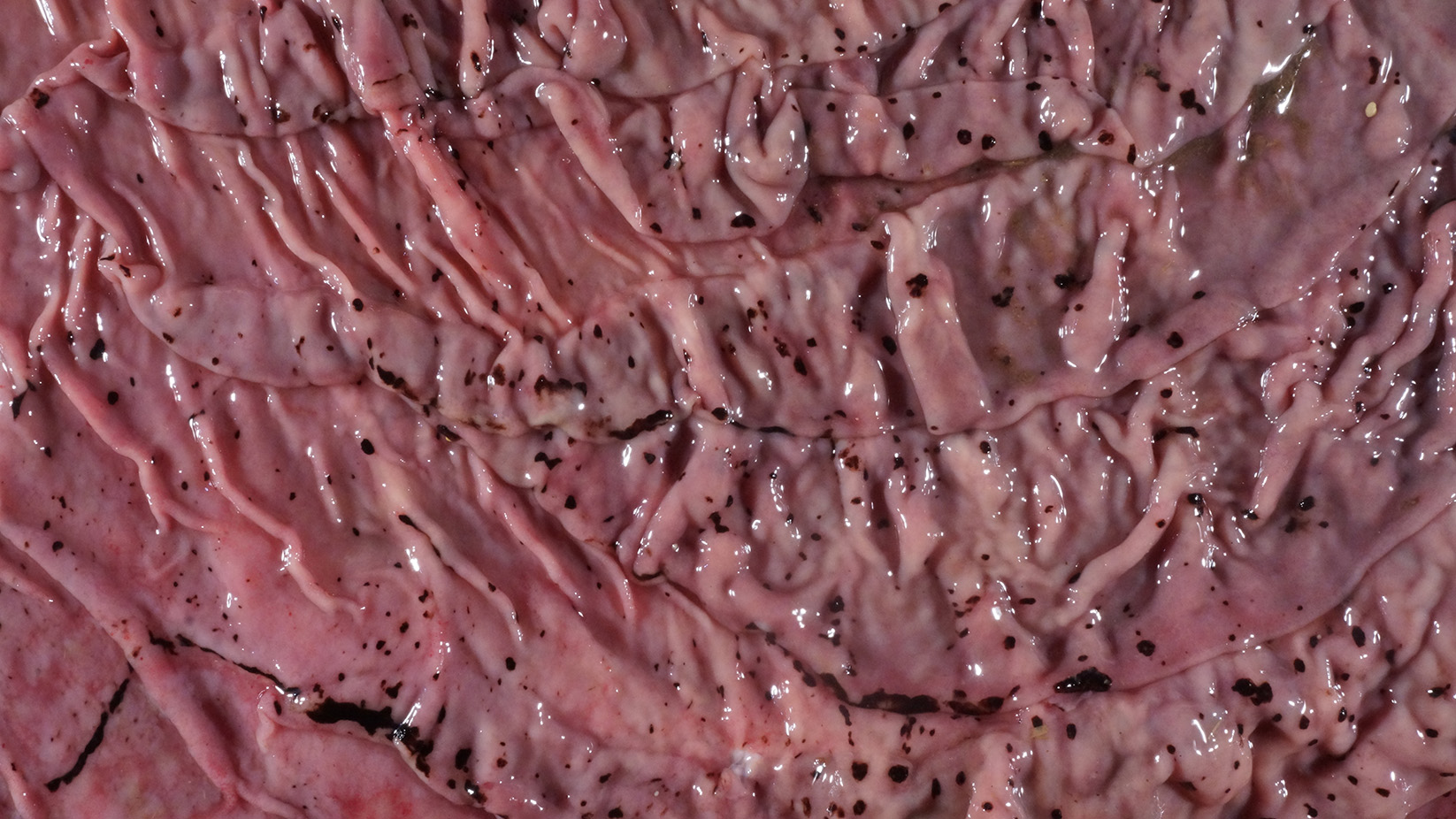
Alimentary system: At the rostral aspect of the hard palate and at the margins of the buccal mucosa, there were multifocal to coalescing, 5-10mm diameter, dark red, slightly depressed regions with partial to complete loss of the mucosa (erosions and ulcers) as well as multifocal pinpoint erosions/ulcers at the junction of the hard and soft palate. There were numerous, multifocal to coalescing, approximately linear, 1-3mm x 5-10mm, slightly depressed erosions/ulcers that were widely disseminated along the entire length of the mucosal aspect of the esophagus. The abomasal mucosa was diffusely red with numerous, 2-5mm dark red to black erosions/ulcers widely disseminated throughout the mucosal surface affecting approximately 20-30% of the surface area. The small intestinal mucosa was reddened. Multifocally and widely disseminated throughout the mucosal surface of the small intestine, there were small numbers of 3mm diameter, black foci. The colonic and rectal mucosa had multiple red approximately linear regions. There was a large amount of soft to watery intestinal contents.
Laboratory Results:
Molecular Diagnostics: Tissue homogenate was positive for BVDV by PCR.
Microscopic Description:
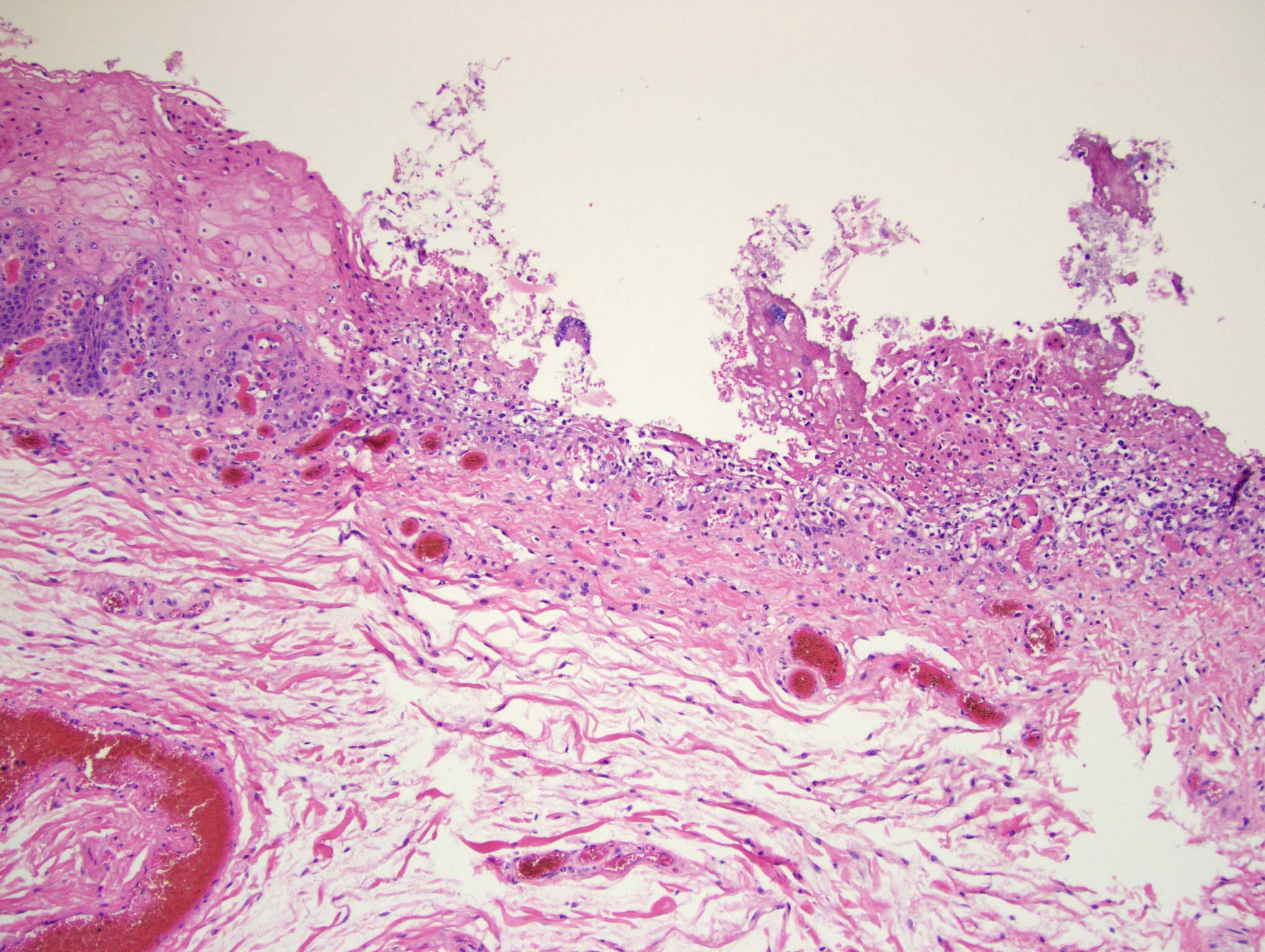
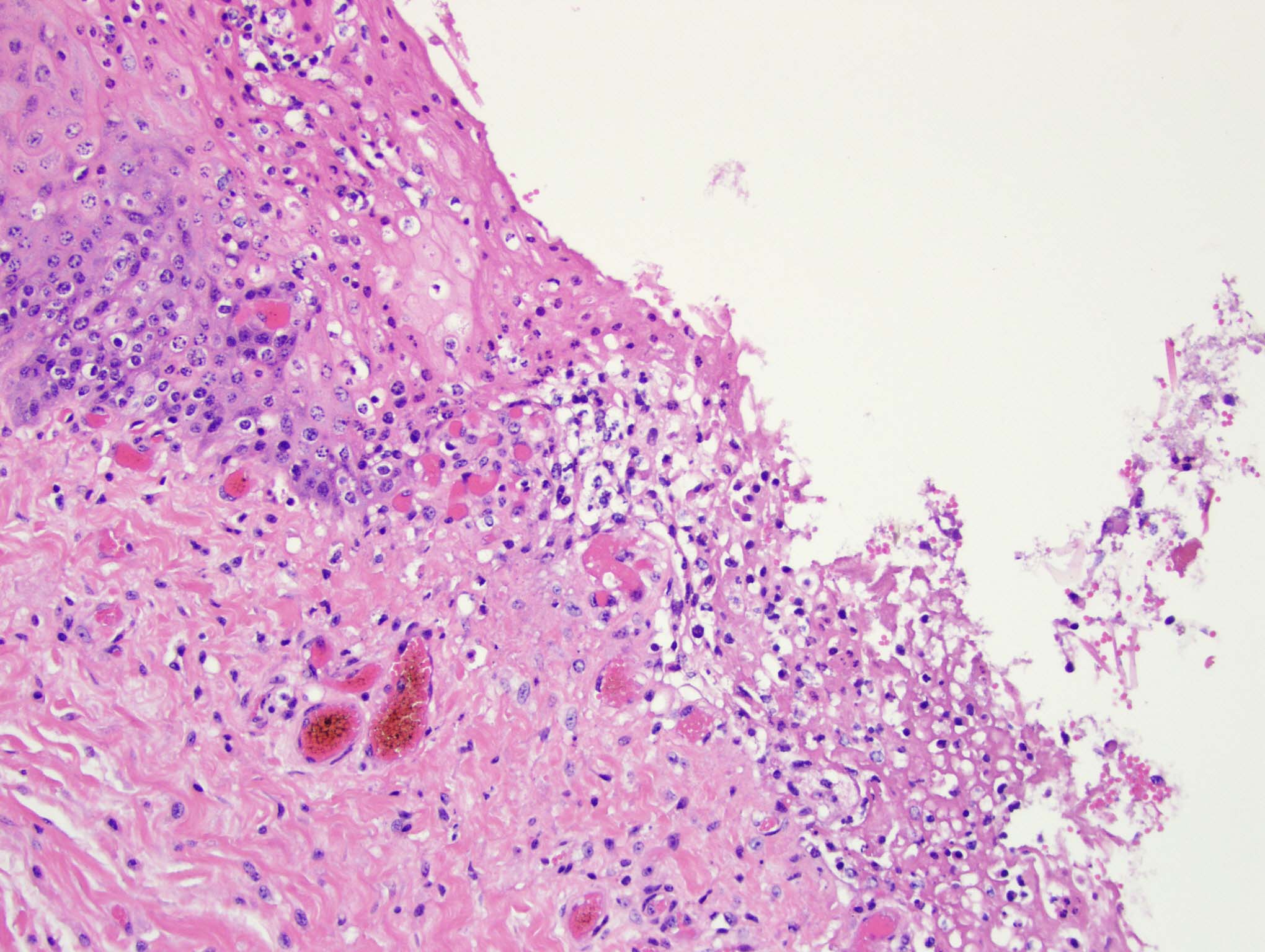
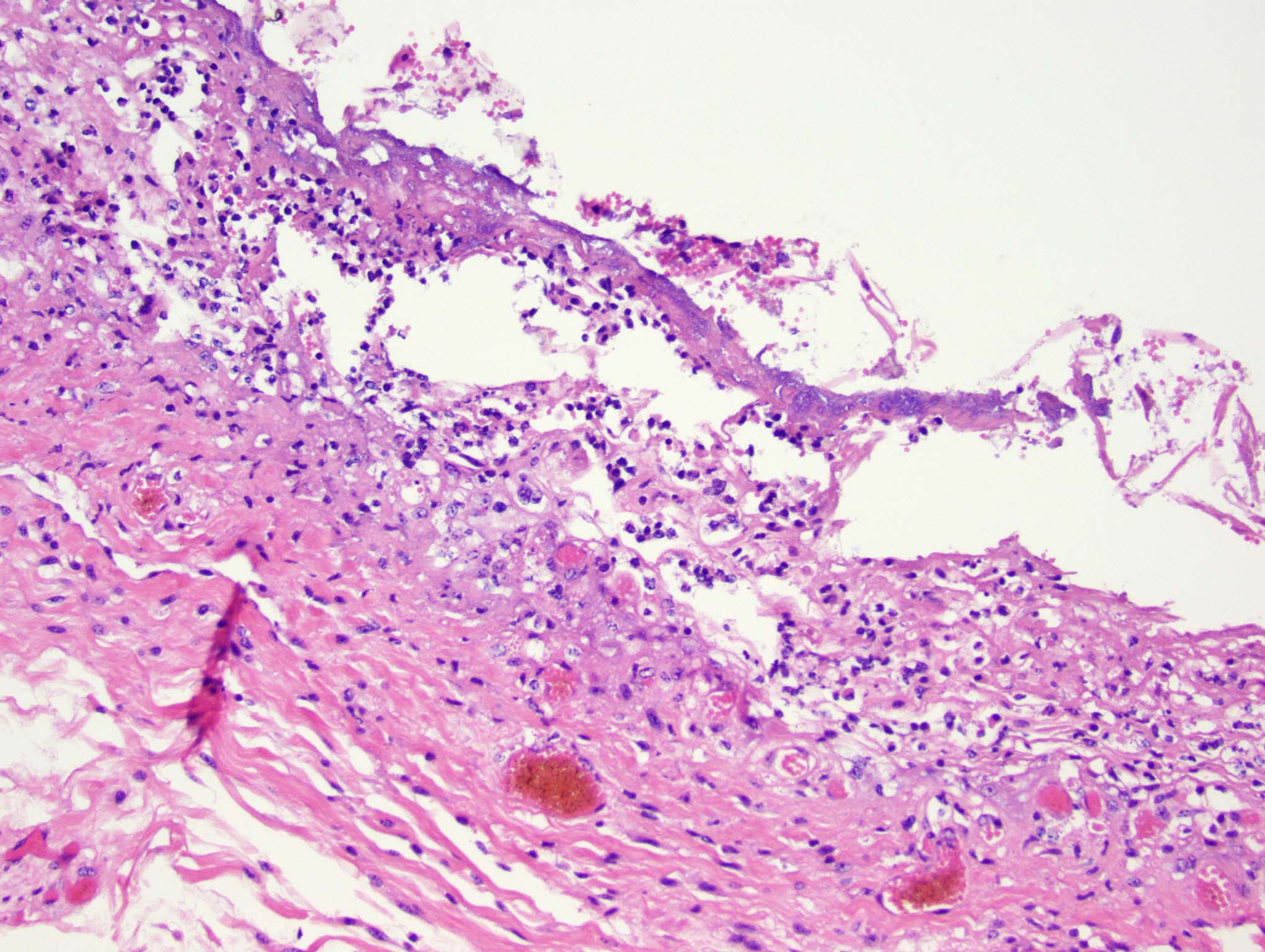
Esophagus: Multifocally, there is partial to complete loss and sloughing of the epithelium (erosion and ulceration) with replacement by necrotic cellular debris, few neutrophils, a small amount of fibrin, few erythrocytes, and occasional bacterial colonies. The adjacent epithelial cells are often swollen and pale (ballooning degeneration) or are shrunken, deeply eosinophilic with pyknotic to karyorrhectic nuclei (necrosis). Multifocally in other regions, the epithelium is degenerate to necrotic. There are small numbers of neutrophils infiltrating the remaining epithelium (transmigration) and occasionally extending into the underlying submucosa forming microabscesses. The mucosal and submucosal blood vessels are congested.
Contributor's Morphologic Diagnosis:
Esophagus: necroulcerative esophagitis, multifocal to coalescing, marked, acute with few neutrophils and rare microabscesses.
Contributor's Comment:
Bovine viral diarrhea virus (BVDV) is an RNA virus that most commonly causes disease in cattle, but it, or closely related viruses, can infect most even-toed ungulates including swine and camelids. 14 BVDV (BVDV-1 and BVDV-2) is in the genus Pestivirus (family Flaviviridae) which also includes Classical swine fever virus and Border disease virus.14 BVDV has two biotypes including both a noncytopathic (NCP) and cytopathic (CP) form.14 Additionally, there is a more recently identified species of pestivirus which has been classified as BVDV-3, also known as HoBi-like or atypical pestivirus.14
There are multiple varying clinical presentations of BVDV in juvenile to adult cattle ranging from mild clinical signs (fever, anorexia, lethargy) to severe clinical signs and death.14 A severe acute form of BVDV is termed BVD type 2 (often caused by BVDV-2) and presents with a fever, sudden death, diarrhea, or pneumonia.14 A thrombocytopenic form of BVDV has been described.14 These varying forms are not mutually exclusive and often present with overlap. Fetal infections, which vary clinically by time of gestation, can occur when an immunocompetent, seronegative dam is infected. If the dam is infected during approximately the first 4 months of gestation by a NCP form of BVDV that crosses the placenta, the fetus may die leading to resorption, mummification, abortion, develop congenital abnormalities, or survive leading to birth of a persistently infected (PI) calf (typically 42-125 days gestation).8,14,16
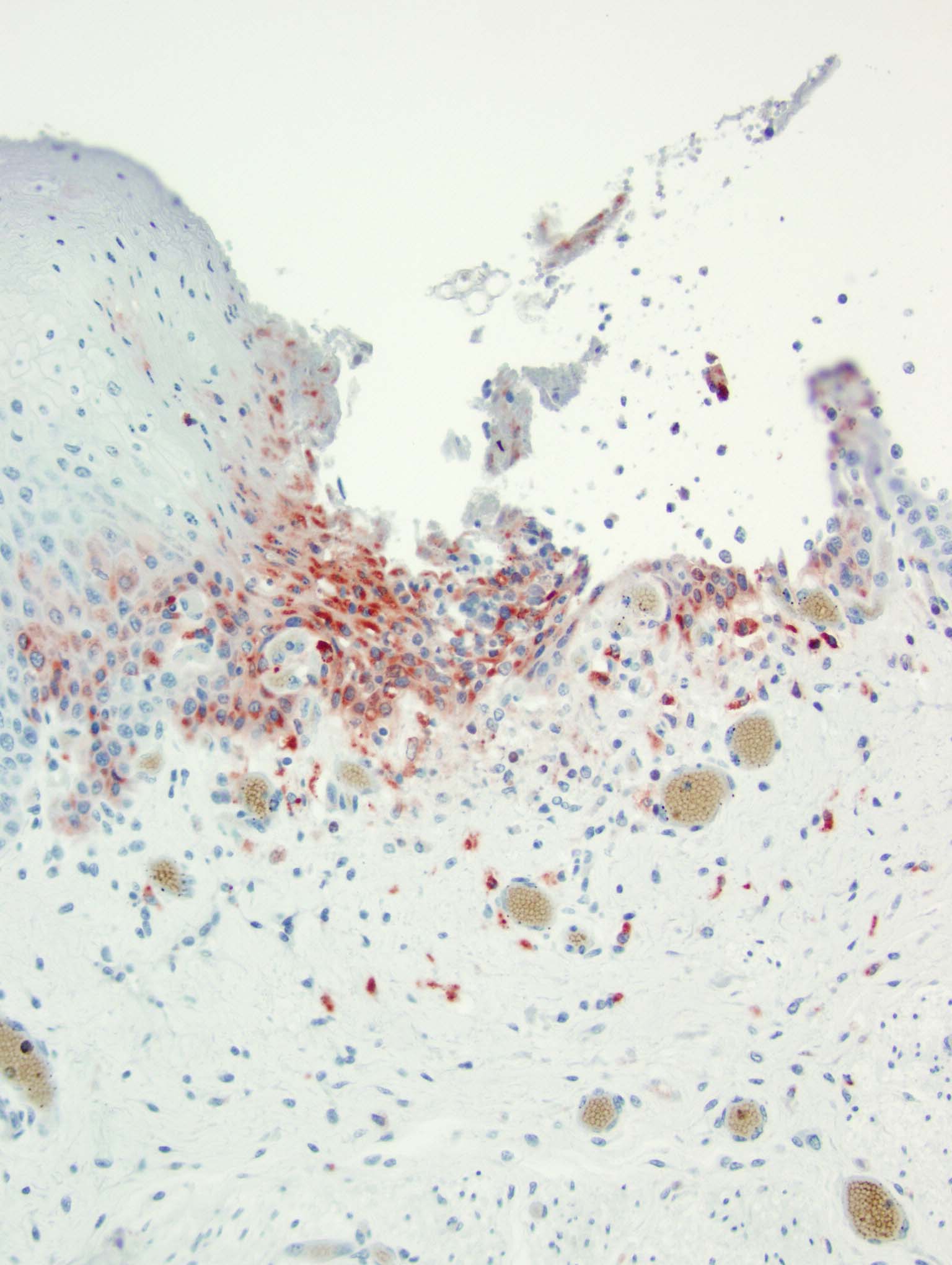
At birth PI calves can appear completely normal or have non-specific clinical signs (poor doing, rough hair coat), or are undersized. PI calves remain viremic throughout life and shed large amounts of virus.14 Due to the timeframe of fetal infection and immune system development, these calves are seronegative but antigen positive, and viral antigen can be identified with immunohistochemistry in a variety of tissues.14 PI calves must be differentiated from acutely infected calves by the absence of lesions in the presence of antigenic positivity.14 PI calves are vulnerable to mucosal disease.14
Mucosal disease is a syndrome whereby PI calves become infected with a CP biotype that is similar to the NCP biotype of original fetal infection or when the NCP biotype of the persistent infection mutates.14 Evidence has shown that vaccination with a modified-live BVDV can also lead to mucosal disease in PI calves.14 Due to the immunotolerance created by the timing of the fetal infection, the calf is unable to elicit an immune response to the CP BVDV leading to an overwhelming infection and destruction of mucosal epithelial cells.8,14,16
Histopathologic features of mucosal disease and acute BVDV include epithelial necrosis with erosions and ulcerations throughout the alimentary tract often including esophagus, rumen, reticulum, abomasum, and intestine.14 The most characteristic histopathologic features of BVDV are found in the intestine and include destruction of the crypts of Lieberk?hn with luminal dilation by mucus along with lysis of the Peyer's patches and associated overlying mucosal inflammation.14 Differential etiologies for the histologic findings of epithelial necrosis with erosions and ulceration in the alimentary tract include malignant catarrhal fever, rinderpest, and vesicular diseases; however, the intestinal changes are only similar in rinderpest.8,14 Osteopetrosis has also been identified in PI calves.15
Due to the economic importance of BVDV, there have been a few recent studies completed to streamline diagnosis and understand pathogenesis and lesions. As PI animals are the main concern for spread of disease, better detection strategies for rapid identification were warranted. According to Brodersen, the use of ear notches with immunohistochemistry has enabled quick identification of PI animals to assist with on-farm control strategies.4 As previously described, mucosal disease typically affects the entire alimentary tract; however, a recent report describes a mucosal disease outbreak with lesions restricted to the upper alimentary tract and skin (interdigital) with no lesions in the intestine, which may pose a challenge when differentiating from vesicular diseases.3 In order to more fully understand the pathogenesis of mucosal disease, varying apoptotic pathways were evaluated by Hilbe et al.10 It was found that caspase-3 and caspase-9 (intrinsic pathway) are more strongly expressed in mucosal disease lesions while caspase-8 (extrinsic pathway) was not.10
Contributing Institution:
Veterinary Diagnostic Laboratory, University of Minnesota, www.vdl@umn.edu
JPC Diagnosis:
Esophagus: Esophagitis, ulcerative, acute, multifocal.
JPC Comment:
Bovine viral diarrhea was first described as a new disease of cattle in 1946 by researchers in New York. Subsequent work in cell cultures allowed for isolation of the virus and vaccine development in the 1960s.6 The proclivity of the virus to survive in culture and the spread of the infection in pregnant cows lead to contamination of numerous ruminant cell cultures uncovered in the 1980s. Viral contamination of cell cultures can occur through the addition of infected fetal bovine serum or, less commonly, by using infected bovine fetal tissue for the initial culture.2,9 Such viral contamination compromises research studies and in several instances has led to contamination of vaccine stocks in both veterinary patients and humans. Early poliovirus vaccines produced on rhesus monkey kidney cell cultures were contaminated with simian polyomavirus SV40, a virus endemic to rhesus macaques and carcinogenic in humans.5 This led to widespread exposure of the human population between 1955 and 1963 and even later in some countries. Strict controls on international trade have been implemented and new improved protocols require irradiation of serum prior to addition to cell cultures.9 Additionally, technological advances now allow for detection and sequencing of all nucleic acids within a culture.9 It was through these detection methods that HoBi-like pestivirus (HoBiPeV) was first identified in 2004 when it was isolated from contaminated bovine fetal serum from Brazil.11
As the contributor described, bovine viral diarrhea virus has two traditional genotypes, BVDV-1 and BVDV-2. HoBiPeV can cause the produce the same spectrum of disease as the typical BVDV genotypes and has now been reported in South America, Europe, and Asia.1,11 In a recent report of HoBi-like pestivirus in feedlot steer in Argentina, the virus was associated with bronchointerstitial pneumonia in one animal and fibrinosuppurative bronchopneumonia in another animal coinfected with Mannheimia hemolytica.11 This report is suggestive of HoBiPeV's role in development of bovine respiratory disease complex, similar to that of BVDV. This study also demonstrated HoBiPeV's potential cross-reactivity against BVDV immunohistochemical stains, which is attributed to the highly-conserved viral glycoprotein GP48 present in all three species. Current antigenic and serologic tests are less or ineffective at detecting HoBiPeV, and there is limited cross protection between strains in commercially available BVDV vaccines.7,11
Since 2000, the list of known pestiviruses has grown to include several additional pathogens of cloven-hoofed animals, including atypical porcine pestivirus and Bungowannah virus in pigs, Aydin-like pestivirus in sheep and goats, and pronghorn antelope virus. Additionally, a number novel pestiviruses of questionable pathogenicity have been isolated from non-ungulate species, including pangolins, rodents, bats, and harbor porpoises.12 With this growing list, there is a proposed revision of Pestivirus taxonomy, with each species denoted by a single letter. Under the new taxonomy, BVDV-1 is designated Pestivirus A, BVDV-2 is Pestivirus B, and HoBiPeV is Pestivirus H.13
References:
- Balasuriya UBR, Reisen W. Chapter 29: Flaviviruses. In: MacLachlan NJ, Dubovi EJ eds. Fenner's Veterinary Virology. 5th ed. Boston, MA: Academic Press, 2017: 525-546.
- Barrat-Boyes S. Golde WT. Chapter 4; Antiviral Immunity and Virus Vaccines. In: MacLachlan NJ, Dubovi EJ eds. Fenner's Veterinary Virology. 5th ed. Boston, MA: Academic Press, 2017: 79-104.
- Bianchi MV, Konradt G, de Souza SO, et al. Natural Outbreak of BVDV-1d-Induced Mucosal Disease Lacking Intestinal Lesions. Vet Pathol. 2017;54:242-248.
- Brodersen BW. Bovine Viral Diarrhea Virus Infections: Manifestations of Infection and Recent Advances in Understanding Pathogenesis and Control. Vet Pathol. 2014;51:453-464.
- Carbone M, Gazdar A, Butel JS. SV40 and human mesothelioma. Transl Lung Cancer Res. 2020 Feb;9(Suppl 1):S47-S59.
- Coffey LL. Chapter 1:The Nature of Viruses. In: MacLachlan NJ, Dubovi EJ eds. Fenner's Veterinary Virology. 5th ed. Boston, MA: Academic Press, 2017:1-16.
- Decaro N. HoBi-Like Pestivirus and Reproductive Disorders. Frontiers in Vet Sci. 2020;7:1-5.
- Gelberg HB. Alimentary System and the Peritoneum, Omentum, Mesentery, and Peritoneal Cavity. In: Zachary JF. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, MO: Elsevier; 2017: 395-397.
- Heidner H. Chapter 2: Virus Replication. In: MacLachlan NJ, Dubovi EJ eds. Fenner's Veterinary Virology. 5th ed. Boston, MA: Academic Press, 2017:17-46.
- Hilbe M, Girao V, Bachofen C, et al. Apoptosis in Bovine Viral Diarrhea Virus (BVDV)-Induced Mucosal Disease Lesions: A Histological, Immunohistological, and Virological Investigation. Vet Pathol. 2012;50:46-55.
- Margineda CA, Ferreyra FM, Masnyj F, et al. HoBi-like pestivirus in 2 cases of fatal respiratory disease of feedlot cattle in Argentina. J Vet Diagn Invest. 2022; 34(4):693-698.
- Postel A, Smith DB, Becher P. Proposed Update to the Taxonomy of Pestiviruses: Eight Additional Species within the Genus Pestivirus, Family Flaviviridae. Viruses. 2021 Aug 4;13(8):1542.
- Smith DB, Meyers G, Bukh J, et al. Proposed revision to the taxonomy of the genus Pestivirus, family Flaviviridae. Gen. Virol. 2017;98:2106:2112.
- Uzal FA, Plattner BL, and Hostetter JM. Alimentary System. In: Maxie MG. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Missouri: Elsevier, 2016: Vol 2: 122-139.
- Webb BT, Norrdin RW, Smirnova NP, et al. Bovine Viral Diarrhea Virus Cyclically Impairs Long Bone Trabecular Modeling in Experimental Persistently Infected Fetuses. Vet Pathol. 2012;49:930-940.
- Zachary JF. Mechanisms of Microbial infections. In: Zachary JF. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, MO: Elsevier; 2017: 200-201.