WSC 22-23
Conference 17
Case II:
Signalment:
3½ month-old, intact female, Siberian Husky, Canis lupus familiaris, dog.
History:
This puppy was initially presented to our institution's veterinary teaching hospital with a history of anorexia, diarrhea and mild intermittent fever of 10 days duration. It had been vaccinated against CDV, CPV-2, CAV-1, Leptospira spp. and Bordetella bronchiseptica at 2 and 3 months of age, and administered anthelminthics (fenbendazole). At presentation, moderate dyspnea was observed and treated with antibiotics, anti-inflammatory drugs, IV fluids and oxygen. Hematology revealed moderate lymphopenia. The respiratory signs gradually worsened over the next 5 days, with seizures developing within 3 days after presentation; the seizures were well controlled with diazepam. Considering the progressive deterioration of the animal's condition, the owners elected for euthanasia. Littermates were unaffected.
Gross Pathology:
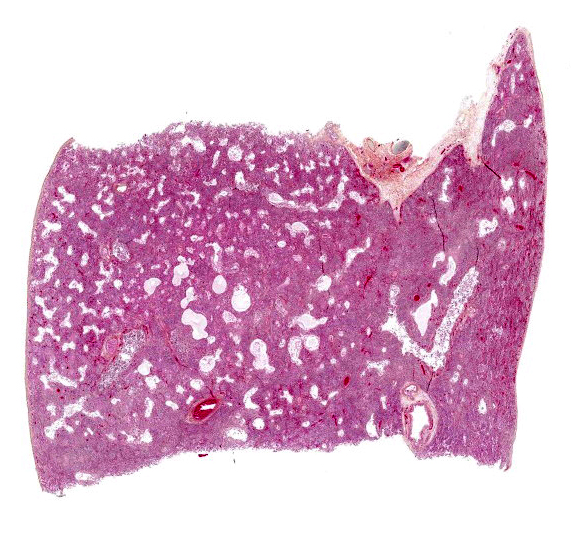
Body condition was assessed as 3/9. The lungs failed to collapse when the thorax was opened, and they were diffusely pale red to purplish with a rubbery to firm texture (interstitial pneumonia); there was no exudate on cut sections. The only other significant gross lesion was marked thymic atrophy.
Laboratory Results:
PCR for canine distemper virus (CDV) on lung samples was positive (Ct: 17.00)
Microscopic Description:
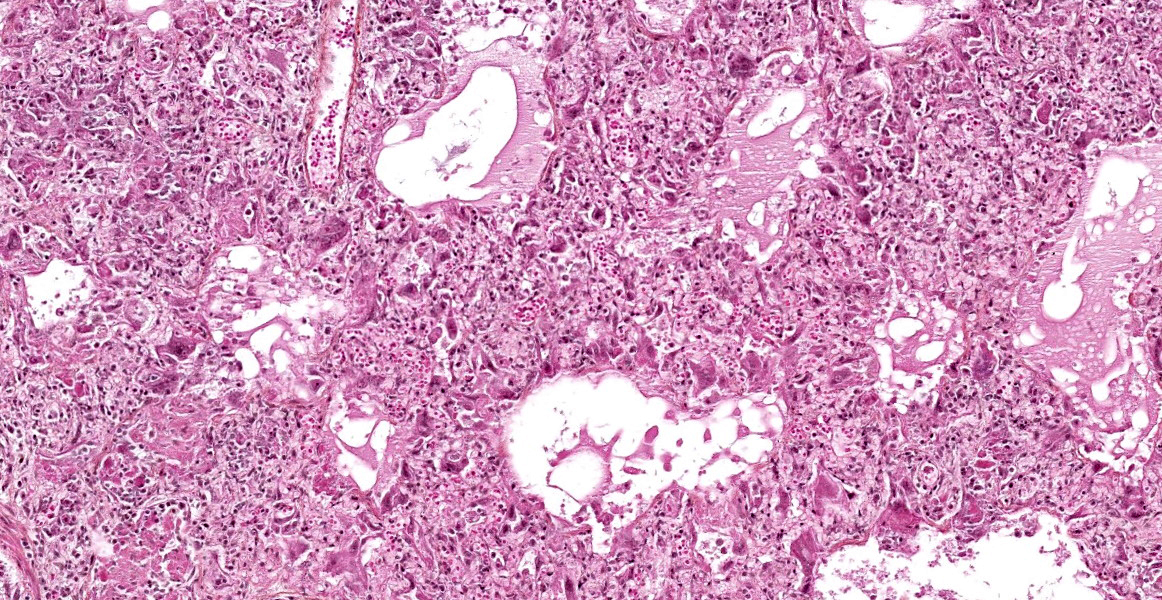
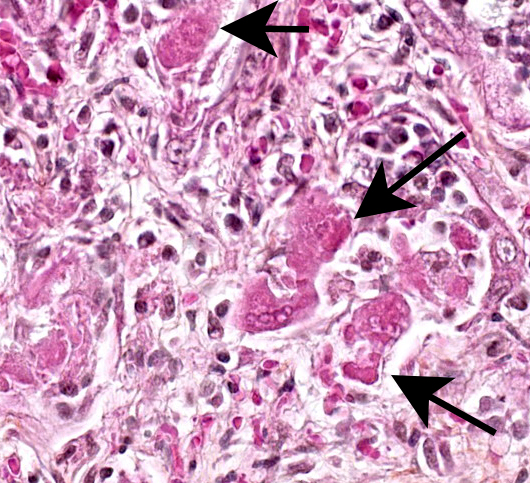
The lesions are similar in all pulmonary lobes (only one section submitted). Diffusely, the pulmonary alveoli are lined by pleomorphic squamous to polygonal cells (type II pneumocytes), many of them multinucleated (syncytia). Multifocally and to variable degrees within the alveolar lumina, there are macrophages and desquamated pneumocytes with protein-rich edema and, to a lesser degree, fibrin and/or necrotic debris; small numbers of neutrophils are occasionally present. Minimal, mainly lymphoplasmacytic interstitial inflammation is present in the alveolar septa. Although there is significant post-mortem desquamation, some terminal bronchioles and alveolar ducts can be seen to be lined by squamous to cubic epithelial cells often with amphophilic cytoplasm (regeneration). Numerous variably sized, brightly eosinophilic, intracytoplasmic inclusion bodies are present in type II pneumocytes, alveolar macrophages and, to a lesser degree, bronchiolar epithelial cells.
Other relevant lesions observed in this pup were: 1) severe lymphoid depletion in the thymus, spleen and Peyer's patches, 2) inclusion bodies in the renal pelvic and vesical urothelium, 3) mild multifocal, mainly histiocytic cerebral leptomeningitis with intra-histiocytic inclusion bodies, 4) a few syncytial cells (uncertain cell type) with inclusion
bodies in the ocular trabecular meshwork, and 5) a few small foci of myocardial necrosis associated with vascular fibrinoid necrosis and minimal lymphoplasmacytic inflammation. Inclusion bodies were not found in other organs/tissues (e.g. stomach, conjunctiva…), and there were no lesions in the upper respiratory tract and conjunctiva.
Contributor’s Morphologic Diagnoses:
Marked, diffuse, subacute, proliferative and bronchointerstitial pneumonia with numerous epithelial syncytia (type II pneumocytes) and intracytoplasmic eosinophilic inclusion bodies, consistent with canine distemper virus (CDV) pneumonia.
Contributor’s Comment:
Histopathological observations and PCR results were consistent with systemic canine distemper (CD), characterized by marked pneumonia and lymphoid depletion and minimal CNS and ocular lesions; the myocardial necrosis was also attributed to CDV.2,5 Testing for other pulmonary pathogens (e.g. CPIV-5 or CAV-1 PCR; bacteriology) was not done as the lesions were typical for canine distemper virus (CDV) and there was no evidence of secondary bacterial infection. This case was submitted as an example of a CDV-induced proliferative pneumonia with numerous type II pneumocytes syncytia and inclusion bodies which, when present, are characteristic of the disease.2 Canine distemper is a systemic disease caused by a single-stranded RNA virus in the family Paramyxoviridae, genus Morbillivirus. This genus presently includes, in addition to CDV, the Measles virus (MeV), the Phocine distemper virus (PDV), the Cetacean morbillivirus (CeMV), the Peste des petits ruminants virus (PPRV), the Rinderpest virus (RPV) and the more recently discovered Feline morbillivirus (FeMV); the RPV was eradicated in 2011.3 All morbilliviruses share many virological, clinicopathological and epidemiological characteristics, with the exception of FeMV which is associated with renal tubulointerstitial disease.3,10 Typical morbillivirus-induced disease is characterized mainly by profound and protracted immunosuppression, with respiratory, neurological and/or gastrointestinal signs, depending on virus/host.3 As the range of hosts susceptible to MeV infection is very limited (primates), CDV represents an interesting alternative model for the study of morbillivirus-induced disease such as measles.3,4 The spectrum of hosts susceptible to CDV-induced disease includes all families of the order Carnivora, several seal species, javelinas and several macaque species;1,4 CDV has also been isolated from many other terrestrial mammals and CDV is readily transmitted from one species to another.7 In countries where vaccination is routinely done, CD incidence in dogs is low even though some vaccinated animals can develop the disease (possibly due to different strains).1 However, CD is still very much a threat to domestic dogs and ferrets from countries where vaccination is not widespread and to wildlife in general. In the last decades, the known host range has expanded and several large outbreaks in different wildlife species have been documented; for instance, the much-publicized 1994 outbreak in large felids in Tanzania's Serengeti National Park killed about a third of its lion's population.7
Canine distemper is a highly contagious disease. Transmission of CDV is most frequently by inhalation of aerosols from the respiratory tract or direct contact with oronasal secretions, but all secretions are potentially infective.1,2,7,9 The virus reaches the upper respiratory tract or pulmonary mucosa where it infects macrophages and monocytes and then reaches the tonsils and regional lymph nodes; it is highly lymphotropic. There is a subsequent first viremia and the virus is disseminated to all lymphoid organs/tissues (~2-6 days P.I.), either free or within leukocytes. The virus induces apoptosis in lymphocytes, mainly CD4+ T cells, resulting in lymphoid depletion, lymphopenia and immunosuppression. There is a second viremia in which the virus spreads to epithelial cells in many organs, mainly in the respiratory, gastrointestinal and urinary tracts and to the CNS (~10 days P.I).1,2,7,9 The CDV H-protein (hemagglutinin) is considered the key protein in viral attachment to host cells.3,7,11 It is also essential to cell fusion, responsible for the formation of syncytia which are characteristic of systemic morbilliviral diseases.11 There are two major receptors, both with an immunoglobulin-like variable domain, for CDV on host cells: 1) CD150 or SLAM (signaling lymphocyte activation molecule) on B and T lymphocytes, dendritic cells and macrophages, and 2) nectin-4 (poliovirus-receptor-like-4) on epithelial cells.4,7 The pathogenesis, pathology and clinical signs of the neurological form of CD are variable; it will not be discussed further (see reference 1). The severity of CDV-induced disease depends on virus strain (there is only one serotype), host immunity, age and species; in domestic animals, ferrets are considered the most susceptible species.3 Animals with adequate humoral and cellular immunity will generally clear the infection after the first viremia, within 14 days of infection.1
In susceptible dogs, CD is generally a disease of dogs 3-6 months of age, when passive immunity declines.2,9 The disease can be transmitted in utero (transplacental) in CDV-naive animals, causing abortion, weak puppies and fatal neurological disease in newborn puppies; an unusual case with respiratory, but not neurological disease has been reported in 5-12 days puppies.9 The pathology of CD in dogs is variable, but lesions are mostly found in the CNS (most frequently demyelinating leukoencephalomyelitis), the lower respiratory tract and lymphoid organs (lymphocytolysis and lymphoid depletion);1,2 ocular and nasal discharge is relatively frequent and helpful in the clinical diagnosis of respiratory and/or neurological disease (e.g CD vs rabies). In the lungs, CD may cause minimal to no lesions, typical viral bronchitis/bronchiolitis or bronchointerstitial pneumonia with or without type II pneumocyte proliferation. Syncytial formation is variable and observed in the lungs and occasionally in the CNS;2 it has been reported that the more a CDV strain is attenuated, the more it will induce syncytial formation.11 Inclusion bodies can be found in nearly all organs (epithelial cells, histiocytic cells, astrocytes, neurons…), with or without lesions, and are helpful for diagnosis. They are eosinophilic, mostly intracytoplasmic in epithelial and histiocytic cells, and mostly intranuclear in the CNS (astrocytes and neurons); a study found the tonsils to be the most consistently involved cells, which could be of interest for the ante-mortem diagnosis of CD (biopsy).6 Some dogs have minimal CD-associated lesions, but because of immunosuppression develop opportunistic infections (e.g. bacterial and/or CAV-1 pneumonia, toxoplasmosis, cryptosporidiosis …). Other reported lesions include:
- Pustular dermatitis and nasodigital hyperkeratosis/parakeratosis ("hardpad disease")1,2
- Enamel hypoplasia/dysplasia1,2
- Conjunctivitis ± keratitis, retinitis, optic neuritis2
- Polioencephalomyelitis (can be concomitant with leukoencephalomyelitis) and "old dog encephalitis"1,2
- Myocardial necrosis2,5
- Growth retardation lattice (metaphyseal osteosclerosis)1,2
Contributing Institution:
Faculty of veterinary medicine, Université de Montréal. http://fmv.umontreal.ca/faculte/departements/pathologie-et-microbiologie
JPC Diagnosis: Lung: Pneumonia, bronchointerstitial, proliferative and histiocytic, diffuse, severe, with marked type II pneumocyte hyperplasia and hypertrophy, viral syncytia, and intracytoplasmic and intranuclear inclusions.
JPC Comment:
The contributor provides a thorough review of this classic case of canine distemper virus infection. As previously stated, CDV infects a wide range of host species, and a 2020 Vet Pathol report detailed the first documented cases of CDV infections in sloths.8 The outbreak occurred in Tennessee, and five of eight sloths within the quarantine enclosure died after a short history of lethargy and hyporexia.8 Crusting and ulceration over the tongue, oropharyngeal mucosa were present in three animals.8 All cases had lingual and oropharyngeal mucosal erosions and ulcerations, necrotizing bronchointerstitial pneumonia, multifocal splenic necrosis, random hepatic necrosis.8 Intranuclear and intracytoplasmic inclusions were observed in numerous tissues, including oropharyngeal mucosa, trachea, alveolar macrophages, type II pneumocytes, esophagus, urinary bladder, and hepatocytes.8 IHC confirmed CDV presence in the epithelial cells of the tongue, hepatocytes, and less frequently in the meningeal vessel walls, ependymal cells, and choroid plexus.8 The infection also spread to three kinkajous in the same enclosure who died after developing the same clinical signs. 8
The moderators, Dr. Eric Lee and Dr. Juliana Lee, and conference participants discussed the amount of autolysis in this specimen, as evidenced by sloughing of individualized epithelial cells in the bronchioles, which obscured evidence of epithelial necrosis. Ultimately, the group decided there was enough convincing evidence of bronchiolar involvement to diagnose bronchointerstitial pneumonia.
References:
- Beineke A, Puff C, Seehusen F, et al. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet Immunol Immunopathol. 2009;127(1-2):1-18.
- Caswell JL, Williams KJ. Respiratory system. In: Jubb, Kennedy and Palmer’s Pathology of domestic animals, vol.2. Sixth Edition. Elsevier. St.Louis, Mo, 2016:465-591.
- da Fontoura Budaszewski R, von Messling V. Morbillivirus Experimental Animal Models: Measles Virus Pathogenesis Insights from Canine Distemper Virus. 2016;11;8(10). pii: E274.
- de Vries RD, Ludlow M, Verburgh RJ, et al. Measles vaccination of nonhuman primates provides partial protection against infection with canine distemper virus. J Virol. 2014;88(8):4423-33.
- Higgins RJ, Krakowka S, Metzler AE, et al. Canine distemper virus-associated cardiac necrosis in the dog. Vet Pathol. 1981;18(4):472-86.
- Kubo T, Kagawa Y, Taniyama H, et al. Distribution of inclusion bodies in tissues from 100 dogs infected with canine distemper virus. J Vet Med Sci. 2007;69(5):527-9.
- Loots AK, Mitchell E, Dalton DL, et al. Advances in canine distemper virus pathogenesis research: a wildlife perspective. J Gen Virol. 2017;98(3):311-321.
- Nambulli S, Sharp CR, Acciardo AS, Drexler JF, Duprex WP. Mapping the evolutionary trajectories of morbilliviruses: what, where, and whither. Curr Opin Virol.2016; 16:95-105.
- Pandher K, Podell B, Gould DH, et al. Interstitial pneumonia in neonatal canine pups with evidence of canine distemper virus infection. J Vet Diagn Invest. 2006;18(2):201-4.
- Park ES, Suzuki M, Kimura M, et al. Epidemiological and pathological study of feline morbillivirus infection in domestic cats in Japan. BMC Vet Res. 2016;12(1):228.
- von Messling V, Zimmer G, Herrler G, et al. The hemagglutinin of canine distemper virus determines tropism and cytopathogenicity. J Virol. 2001; 75(14):6418-27.
- Watson AM, Cushing AC, Sheldon JD, et al. Natural Canine Distemper Virus Infection in Linnaeus’s 2-Toed Sloths (Choloepus didactylus). Vet Pathol. 2020; 57(2): 311-315.