Signalment:
Gross Description:
Histopathologic Description:
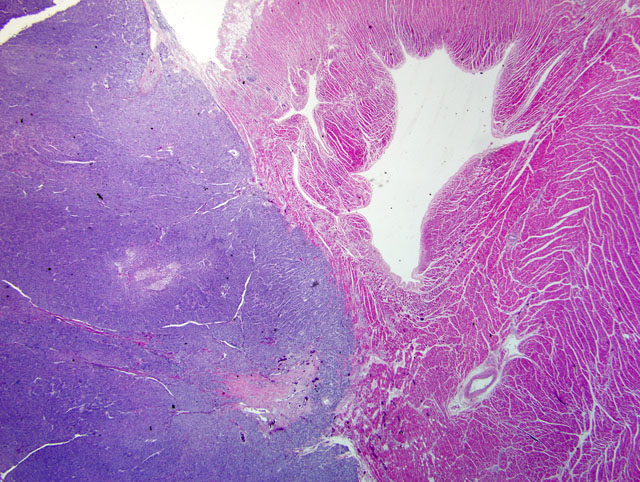
Focally, there is a large aggregate of lymphocytes within the epicardium, and few ectatic vessels contain clusters of neoplastic cells (intracardiac metastasis, not present on every slide). Diffusely, mesothelial cells of the epicardium are enlarged (hypertrophy) and the epicardium contains moderate numbers of inflammatory cells composed of lymphocytes, macrophages, neutrophils and eosinophils, admixed with eosinophilic acellular fibrillar material (fibrin) and occasionally plump fibroblasts. Macrophages multifocally contain golden brown granular pigment (hemosiderin, confirmed with iron stain). Small numbers of inflammatory cells extend into the underlying myocardium. Diffusely, the mitral valve is moderately expanded by increased mucinous matrix and there is mild multifocal interstitial fibrosis within the interventricular septum and left ventricular myocardium. The endocardium of the left atrium is irregular and is diffusely expanded by fibrous connective tissue (endocardial fibrosis). There is also minimal to mild, multifocal myofiber disarray noted within the left ventricular myocardium. Multifocally, the tunica media of small and moderate sized arterioles is thickened by hypertrophic smooth muscle cells (arteriosclerosis).
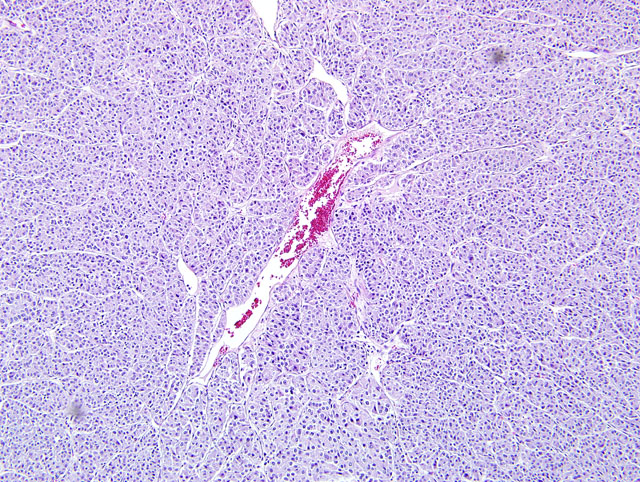
Neoplastic cells exhibit diffuse, strong cytoplasmic staining for synaptophysin and chromogranin A, diffuse weak cytoplasmic staining for neuron specific enolase (NSE), and negative staining for thyroglobulin, cytokeratin, vimentin and calcitonin. Positive and negative controls were run and stained appropriately. Intracardiac metastatic foci stain strongly positive with synaptophysin and chromogranin A. Reticulin stains highlight the connective tissue stroma and enhance the packeted pattern of the neoplasm.
Morphologic Diagnosis:
1. Heart (base between aorta and pulmonary artery): Focal chemodectoma with multifocal epicardial metastasis.
2. Heart: Moderate multifocal chronic ongoing lymphohistiocytic, neutrophilic, fibrinous epicarditis with mesothelial cell hypertrophy, hemosiderosis and fibroplasia.
3. Heart: Moderate diffuse mitral valve myxomatous degeneration (consistent with valvular endocardiosis) and left atrial endocardial fibrosis.
4. Heart: Moderate multifocal arteriolar tunica media hypertrophy (arteriosclerosis), mild multifocal myocardial interstitial fibrosis, and minimal to mild, multifocal ventricular myofiber disarray.
Lab Results:
Condition:
Contributor Comment:
Chemoreceptor tissue is composed of parenchymal cells (chemoreceptors and glomus cells) and sustentacular cells and aids in the regulation of respiration and circulation.(2,4) Chemoreceptors are sensitive to changes in arterial partial pressure of carbon dioxide and oxygen, blood pH and temperature, and stimulation of chemoreceptors may result in adaptive modifications in respiratory rate and/or arterial blood pressure.(2,4,6) In mammals, the chemoreceptor system is part of the parasympathetic nervous system, leading to the terminology non-chromaffin paragangliomas.(6) Neoplasms of the chemoreceptor cells most commonly arise within the aortic or carotid body and less frequently the glomus pulmonale, glandula suprarenalis or in ectopic sites.(2,4,6) Aortic body tumors are more common than carotid body tumors in animals, though carotid body tumors are more often malignant than aortic body tumors.(2) Chemodectomas typically form a single mass or multiple nodules within the pericardial sac near the base of the heart and have been reported to occur between the aorta and pulmonary artery, between the pulmonary artery and left auricular appendage, or below the aorta and right auricular appendage.(2,4-6) The mass in this cat was located at the base of the heart, adjacent to the right atrium and auricle between the aorta and pulmonary artery.
Though chemodectomas are non-functional in animals, clinical signs can be attributed to the neoplasm acting as a space occupying lesion, causing compression of multiple adjacent structures.(2,5,6) The tumors can vary greatly in size (0.5 - 12.5 cm) and are often associated with accumulation of serous, often blood tinged, fluid in the pericardial sac.(2) Pericardial effusion (as noted in this cat) can result from lymphatic invasion of neoplastic cells at the base of the heart or the compression of small pericardial veins, and may cause impairment of normal cardiac function and decompensation, depending on the speed and volume of accumulation.(2,5,6) Obstruction of the thoracic duct and/ or cranial vena cava may lead to pleural effusion, pericardial effusion, ascites, and subcutaneous edema, particularly of the head, neck and forelimbs, while obstruction of blood return to the atria may manifest as systemic congestion. (2,5) Airway and esophageal compression may be clinically evident as dyspnea or coughing and vomiting, respectively.(2) Non-specific signs of systemic illness, including debilitation, anorexia and lethargy may also be noted.(2,5,6) Gross and histologic evaluation of the lungs in this case revealed moderate to severe, diffuse congestion, pulmonary edema and a moderate amount of serosanguinous pleural effusion. These findings are interpreted to be secondary to the compressive effects of the neoplasm. Pericardial effusion is likely secondary to compression, vascular invasion, and hemorrhage within the neoplasm. The associated inflammation in the epicardium of this cat is suspected to be secondary to the pericardial effusion.
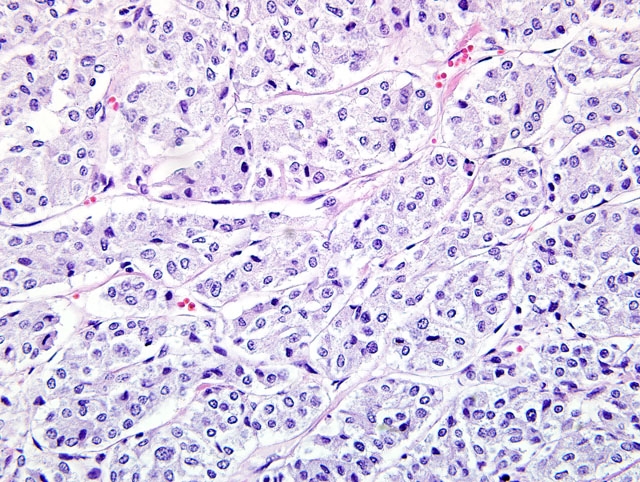
Chemodectomas are typically well demarcated and comprised of round to polygonal cells with indistinct cell borders, scant cytoplasm and central round nuclei with fine chromatin and prominent nucleoli.(4) In our case, positive reticulin and trichrome staining demonstrated the small lobules delineated by fine fibrovascular stroma, which is a typical feature of this neoplasm.(4) Mitoses are rare, and occasionally there may be small amounts of scattered T-lymphocytes throughout the mass.(4,6) Lymphocytes were identified within the neoplasm in this case, but were not further characterized.
In our case, neoplastic cells exhibited diffuse, strong cytoplasmic staining for synaptophysin, chromogranin A, diffuse weak staining for neuron specific enolase (NSE), and negative staining for thyroglobulin, vimentin, cytokeratin and calcitonin, consistent with a diagnosis of chemodectoma. Chemodectomas are known to exhibit positive immunohistochemical reactivity for synaptophysin, chromogranin A and NSE.(4) Synaptophysin is considered to be a specific marker for neuroendocrine tumors because it is not detected in non-neuroendocrine cells or neoplasms.(3,4) Chromogranin positive granules are also typical of neuroendocrine tumors.(4) Occasionally, as noted in this tumor, staining of neoplastic cells for NSE may be weak, hypothesized to result from rapid autolysis of chemoreceptor cells and subsequent loss of immunoreactivity.(4) Neoplastic neuroendocrine cells also commonly exhibit argyrophilic cytoplasmic granules when stained with Churukian-Shenk or Grimelius silver stains (not performed in this case). Differential diagnoses for a heart based tumor in a cat include lymphosarcoma, ectopic thyroid adenoma/carcinoma, ectopic parathyroid chief cell adenoma, and thymoma.(2,6) Tumors of ectopic thyroid tissue may exhibit a similar histologic appearance and immunohistochemistry may be helpful in distinguishing such tumors. Ectopic thyroid tumors should stain positively for cytokeratin and thyroglobulin, while C cell tumors should stain positively for calcitonin.(4)
Because chemodectomas are so rarely identified in cats, biological behavior is essentially unknown.(5) Extrapolating from behavior in other species, aortic body tumors tend to be more benign than carotid body tumors and grow slowly by expansion.(2) Advanced lesions may be associated with neoplastic invasion of the adventitia of the great vessels, atrial myocardium, and as in this case, epicardial vessels, consistent with intracardiac metastasis. (5) Willis et al (2001) reported metastasis in 22% of canine chemodectomas and 2 of 6 (33%) of the reported feline cases.(6) In 2 of 2 cases of feline chemodectoma reported by Tilley et al (1981), tumor emboli were noted in blood vessels, with metastasis to the pericardium, epicardium and myocardium.(5) Multifocal thoracic metastatic foci, with spread to local lymph nodes (mediastinal, sternal) and/or the lungs has also been reported in the cat.(3,5) In this case, there was a site of extracardiac metastasis within tissue that was histologically most compatible with remnant thymic tissue. The highly vascularized stroma frequently associated with chemodectomas may facilitate metastasis via the bloodstream.(5) Chemodectomas may also secrete angiogenic substances, leading to the development of a collateral blood supply that can involve the local vasculature.(6) Long term prognosis is typically guarded to poor, as the tumor is often diagnosed at an advanced stage, though one cat is reported to have lived 13 months post-diagnosis.(5,6) In humans, surgical excision is the treatment of choice and is frequently associated with good success.(6) However, surgical intervention has had limited success in dogs and complete removal is rarely possible.(5,7)
This cat exhibited mandibular prognathism with glossal protrusion, consistent with brachycephalic conformation. Chronic hypoxia is likely involved in the pathogenesis of chemodectoma development, as increased prevalence of this tumor is noted in humans and cattle living at high altitudes, humans with emphysema and brachycephalic dogs. (2,4,6) However, a definitive link with chronically low oxygen tension has not been established in the cat. Ventricular myofiber disarray was minimal to mild. This finding can occur with myocardial hypertrophy, but echocardiographic evaluation revealed that wall thickness was within normal limits. In the absence of additional gross or histologic evidence of hypertrophic cardiomyopathy, the clinical significance of this lesion is not known. The cause for the left atrial endocardial fibrosis is unknown. Mitral insufficiency secondary to valvular myxomatous degeneration is a possible consideration; however, mitral valve abnormalities were not indicated on the echocardiography report.
This cat also had multiple cysts in the kidneys, which may be consistent with polycystic kidney disease, an autosomal dominant congenital disease of a variety of species and breeds of animals, including Persian cats. Mutations in PKD-1 and/or PKD-2 genes leading to altered function of related proteins polycystin-1 and polycystin-2. Polycystin-1 is involved in normal cell proliferation and apoptosis pathways and mutations allow cells either to enter a differentiation pathway that results in tubule formation or to become susceptible to apoptosis, both of which contribute to tubular cyst formation, while polycystin-2 is involved in plasma membrane calcium channels. As cysts enlarge, they compress adjacent parenchyma, leading to secondary changes (compression necrosis and fibrosis of tubules and glomeruli) and when extensive regions are polycystic and impaired, there may be clinically evident renal dysfunction. In this case, the renal cysts are a likely contributor to the chronic renal disease noted in the history. Congenital forms of polycystic kidney disease are also often associated with cystic bile ducts and bile duct proliferation in the liver (as noted in this case) and/or pancreatic cysts (not seen in this cat). In addition, congestion and degenerative changes were noted within the centrilobular regions of the liver, likely secondary to right-sided heart insufficiency due to compression of the heart by the mass.
JPC Diagnosis:
1. Heart base: Chemodectoma.
2. Heart, epicardium: Epicarditis, fibrinous, diffuse, chronic, mild.
3. Heart, atrioventricular valve: Degeneration, fibromyxomatous, focally extensive, mild.
Conference Comment:
References:
2. Capen CC: Endocrine glands. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 3, pp. 425-428. Saunders Elsevier, Philadelphia, PA, 2007
3. Davis WP, Watson GL, Koehler LK, Brown CA: Malignant cauda equina paraganglioma in a cat. Vet Pathol 34: 243-246, 1997
4. Paltrinieri S, Riccaboni P, Rondena M, Giudice C: Pathologic and immunohistochemical findings in a feline aortic body tumor. Vet Pathol 41:195-198, 2004
5. Tilley LP, Bond B, Patnaik AK, Liu S: Cardiovascular tumors in the cat. J Am Anim Hosp Assoc 17:1009-1021, 1981
6. Willis R, Williams AE, Schwarz T, Paterson C, Wotton PR: Aortic body chemodectoma causing pulmonary oedema in a cat. J Small Anim Pract 42:20-23, 2001