WSC 22-23:
Conference 4 Case 2
Signalment:
Twelve years old, neutered female, domestic shorthair cat (Felis catus)
History:
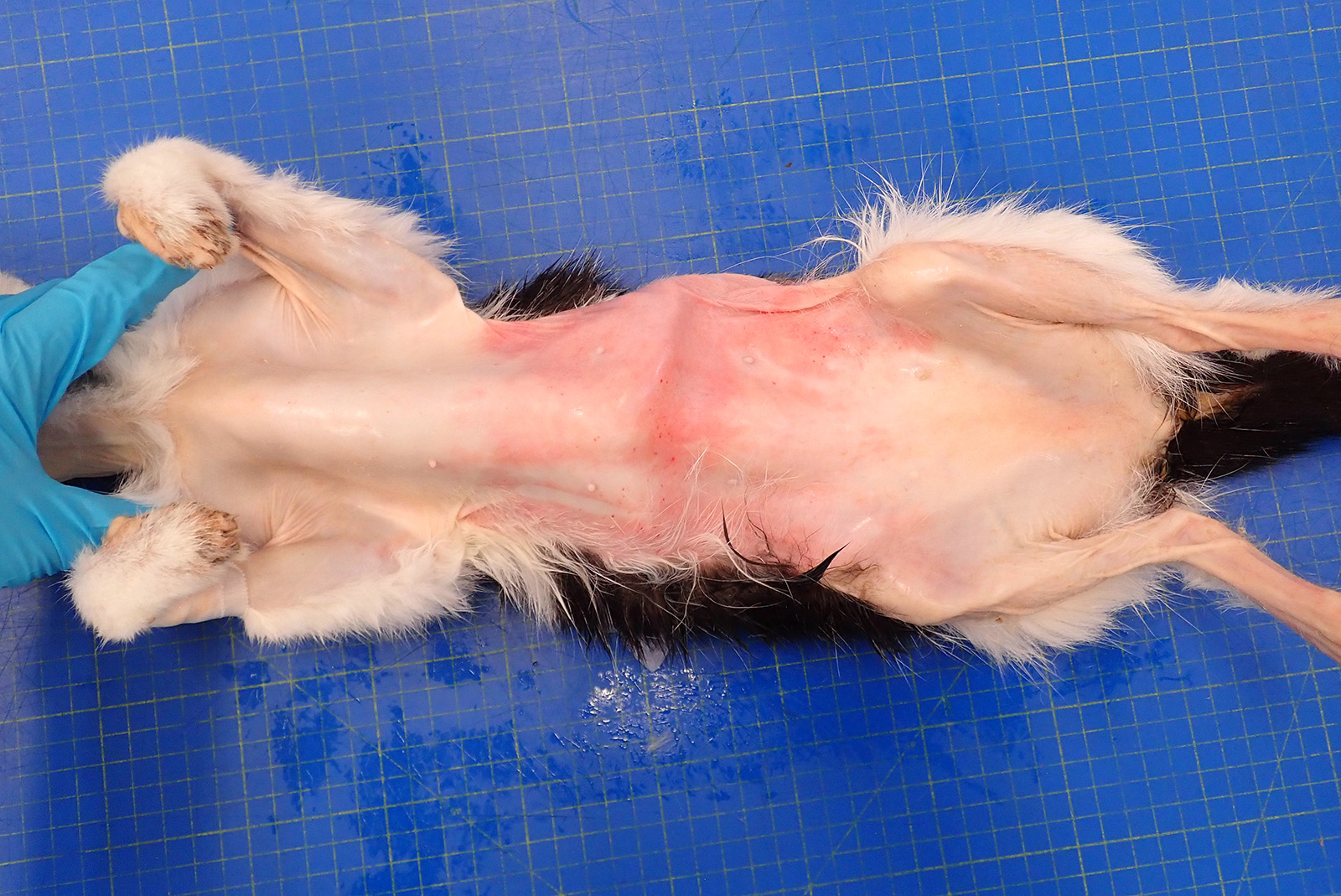
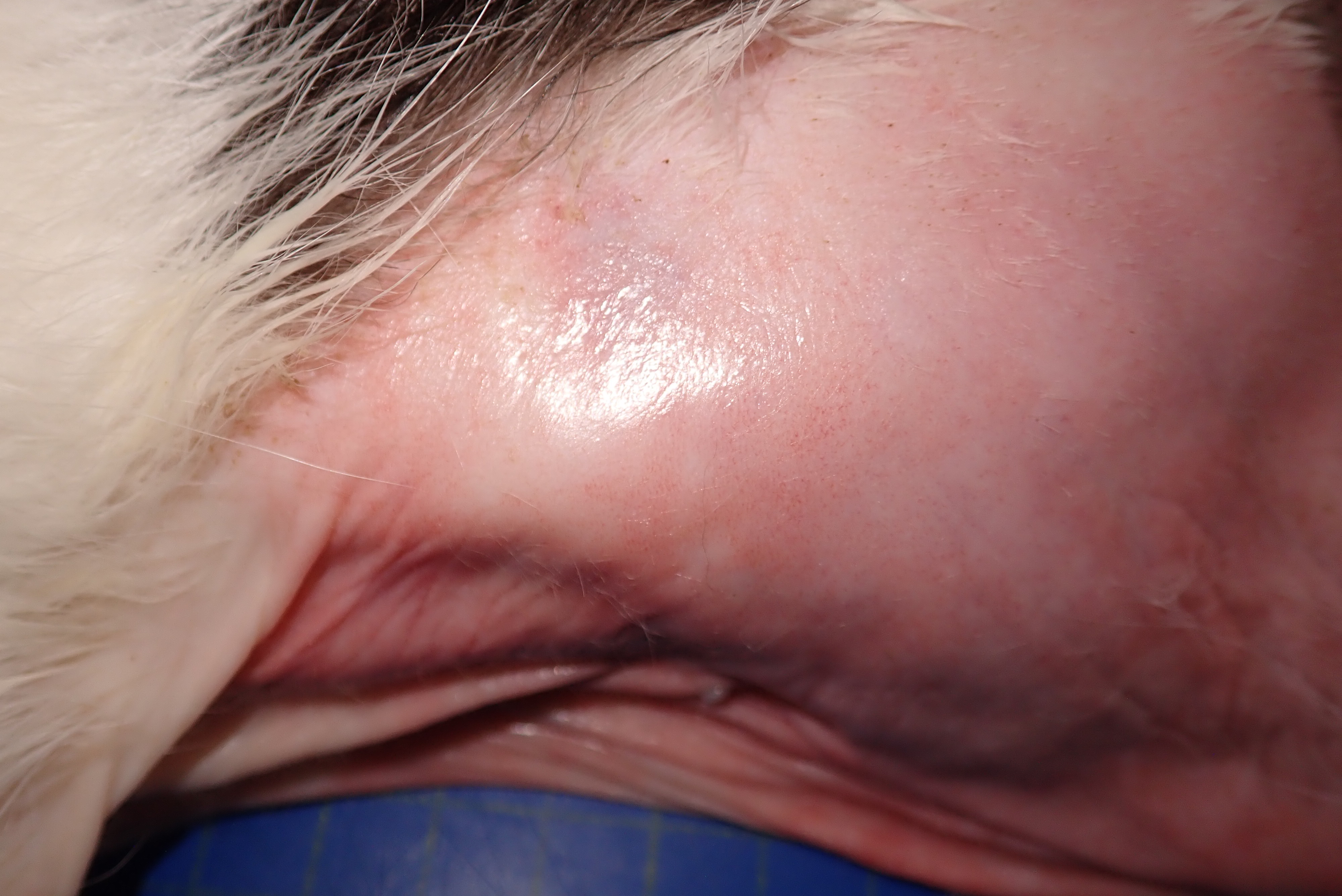
The cat presented with a two month history of anorexia and weight loss. On presentation there was severe alopecia and erythema on the ventral thorax, abdomen, neck, perineum and also extended to the medial aspect of the legs to the paws. The peri-ocular and ear pinna skin also presented with a similar change. Due to the characteristic shiny or glistening appearance of the skin, feline paraneoplastic alopecia was initially suspected. Ultrasound examination was performed with multiple masses detected in the liver and small hypoechoic nodule associated with the small intestinal, pancreas and peritoneum (neoplasia considered most likely). As the masses were widespread and the prognosis was poor, the animal was euthanized.
Gross Pathology:
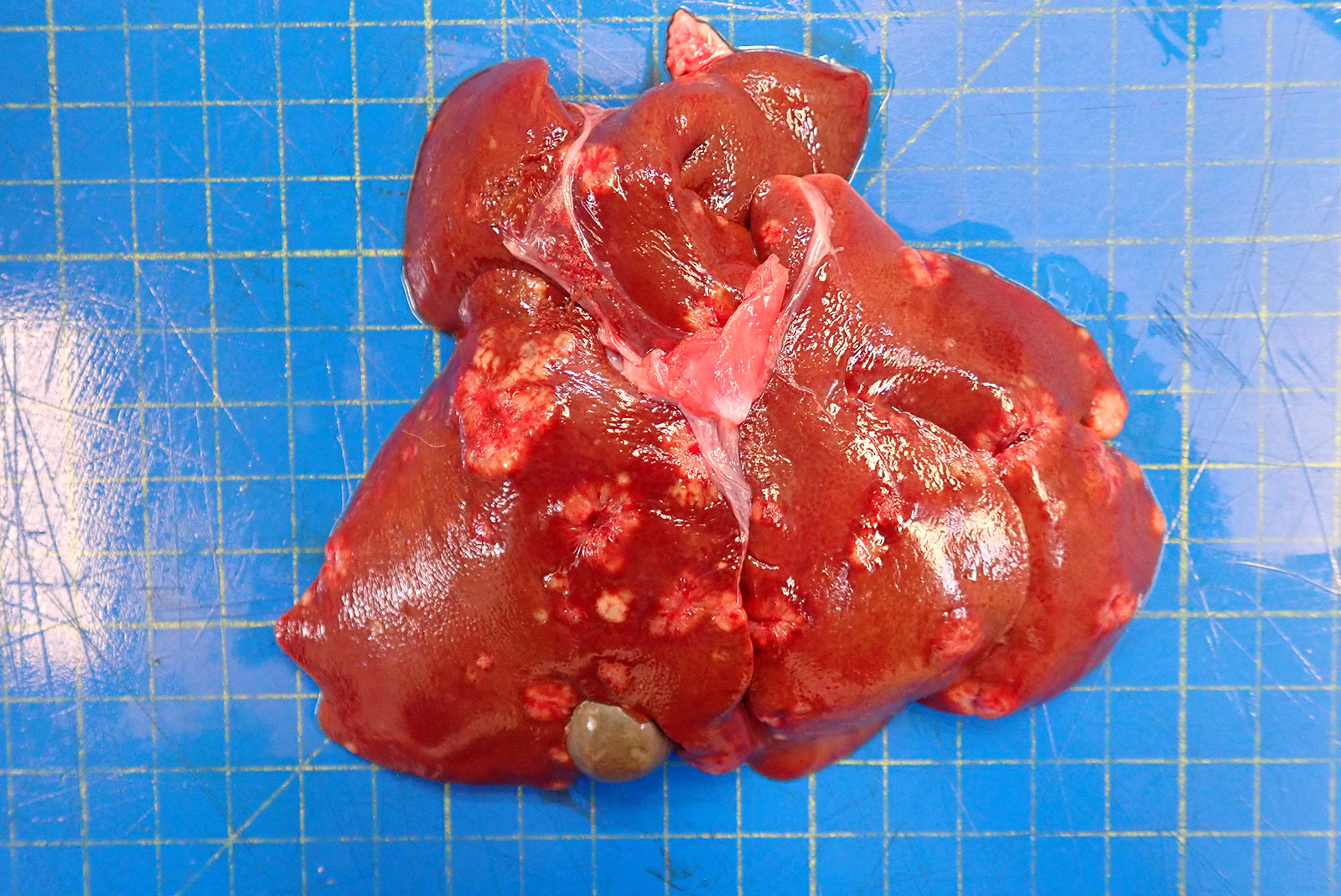
The skin from the sternal to the inguinal regions, extending to the medial antebrachia and to the posterior extremities and partially the lateral abdominal regions, was severely alopecic with a glistening and shiny appearance. Throughout the alopecic skin, poorly demarcated pale red areas (erythema) and rare pale brown crusts were also present. Both periocular and peri-nasal areas were partially alopecic with increased amount of crusts. The right metatarsal paw pad and the right ventral tarsum showed multifocal ulcers. In the liver, scattered throughout all hepatic lobes, there were multifocal to coalescing, 0.2 to 2 cm in diameter, well demarcated, firm, pale tan nodules showing occasional central umbilications.
Laboratory Results:
No laboratory findings reported.
Microscopic Description:
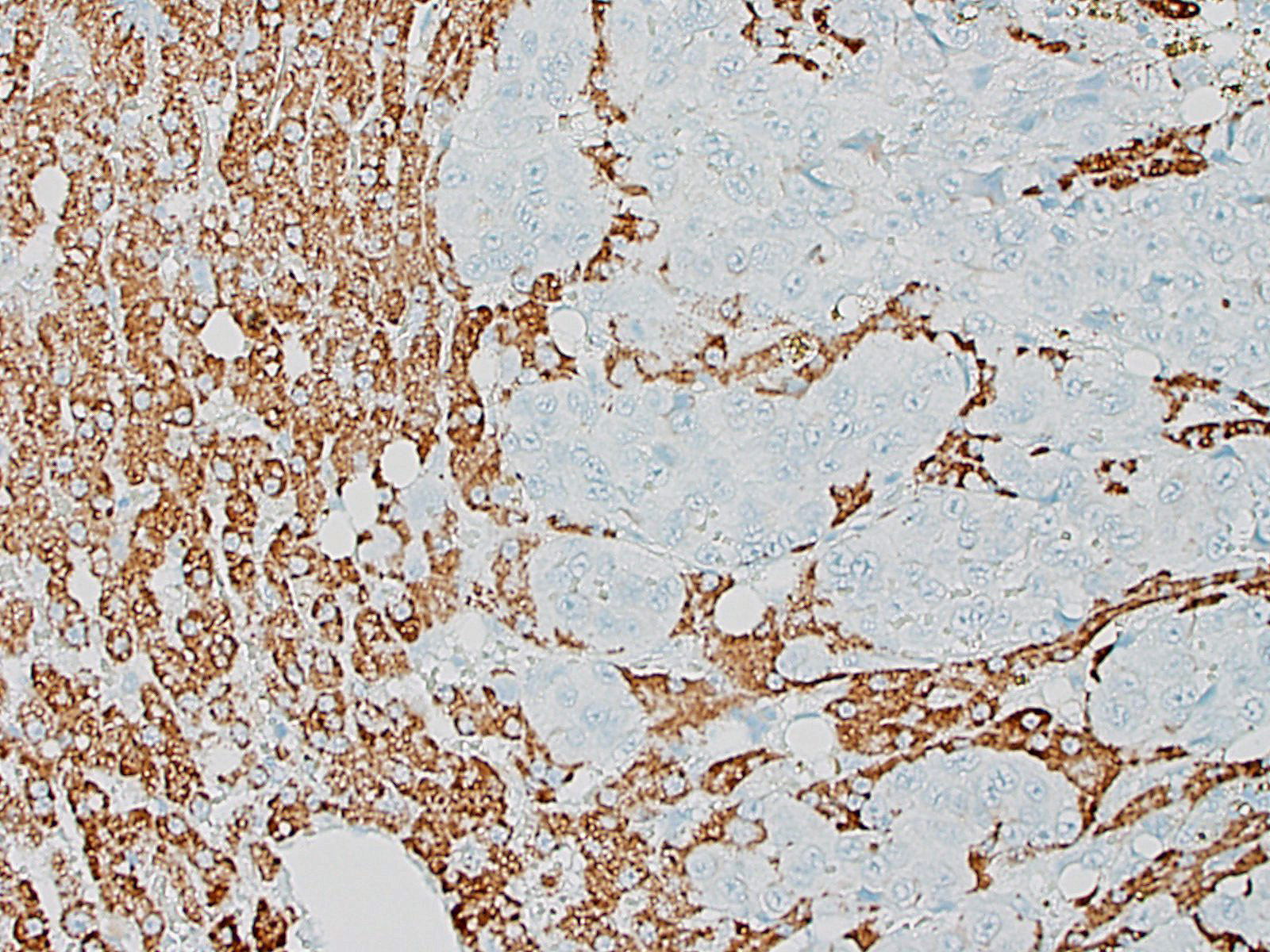
Haired skin (abdomen): Throughout a multifocally mildly hyperplastic epidermis there are multifocal areas of: intracellular edema, spongiosis, and parakeratosis. In rare foci (not present in all slides), the epidermis is restricted to 1-2, poorly defined, thin layers (epidermal atrophy). The hair follicles are diffusely and markedly reduced in size (atrophy and miniaturization) and contain rare intra-luminal eosinophilic trichilemmal keratin (telogen). Adjacent to the atrophied follicles, variably atrophic sebaceous glands and commonly dilated apocrine glands are found. The dermis shows multifocal edema, with mild perivascular to interstitial accumulation of neutrophils, mast cells and extravasated erythrocytes (hemorrhage). Numerous deep dermal and subcutaneous lymphatic vessels (not present in all slides) are markedly dilated by intraluminal accumulation of concentrically arranged, homogenous and acellular eosinophilic material (likely proteinaceous lymph) and rare foamy (activated) macrophages.
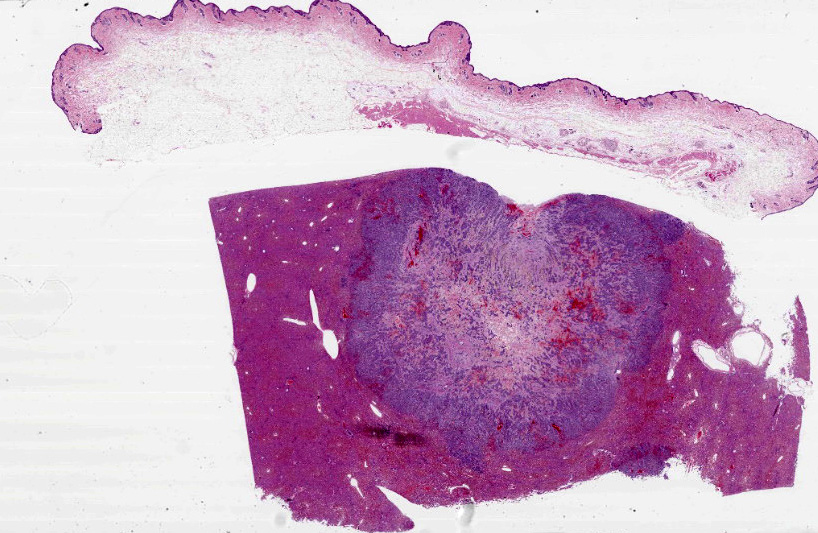
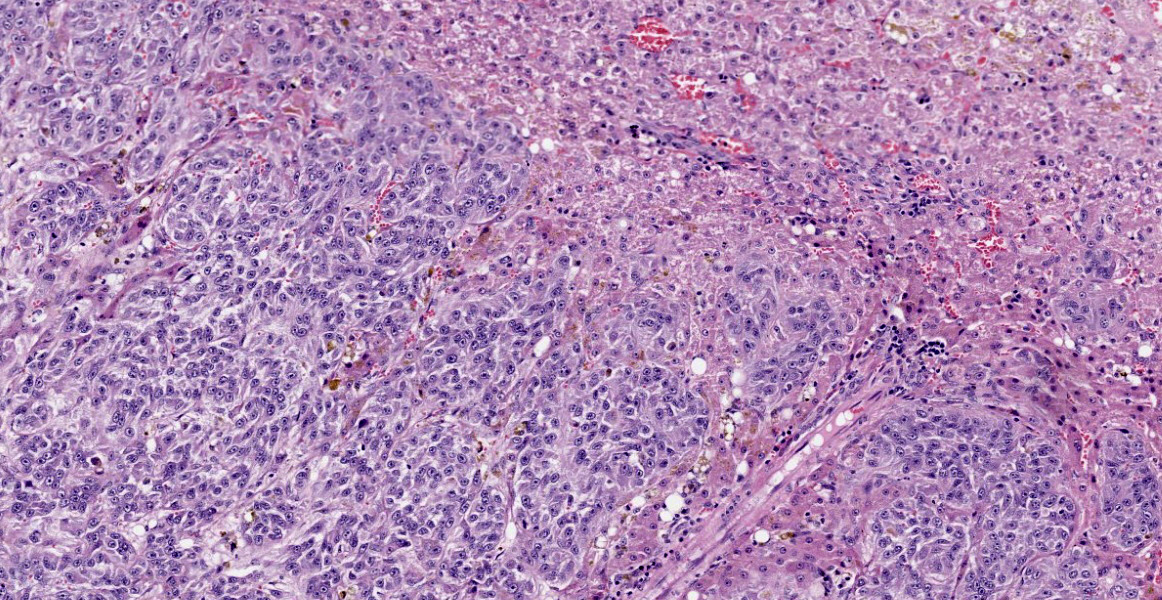
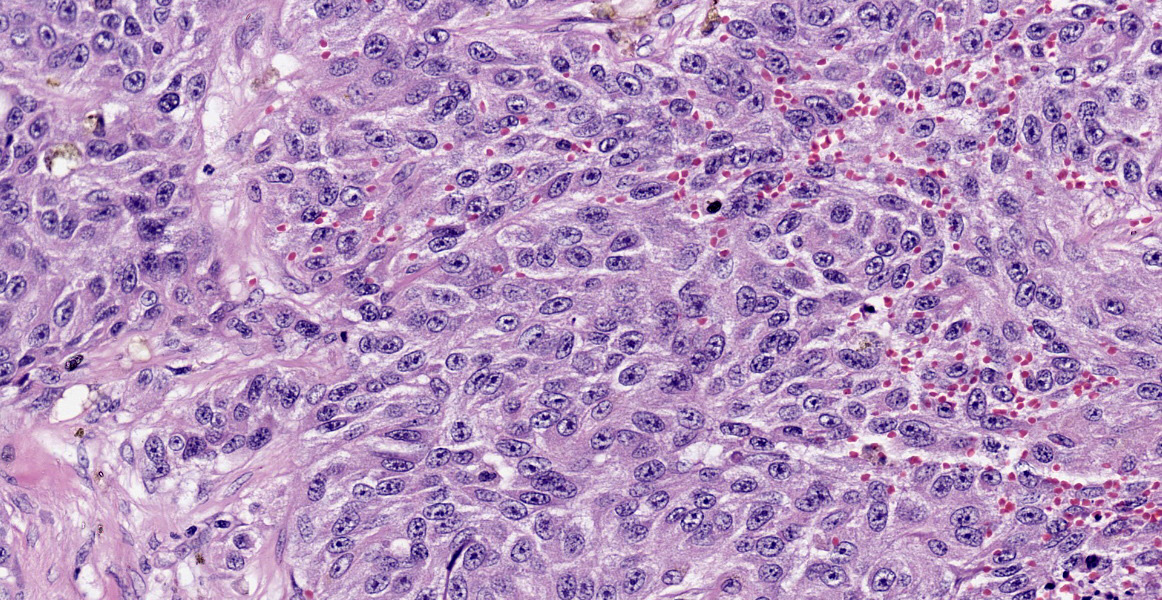
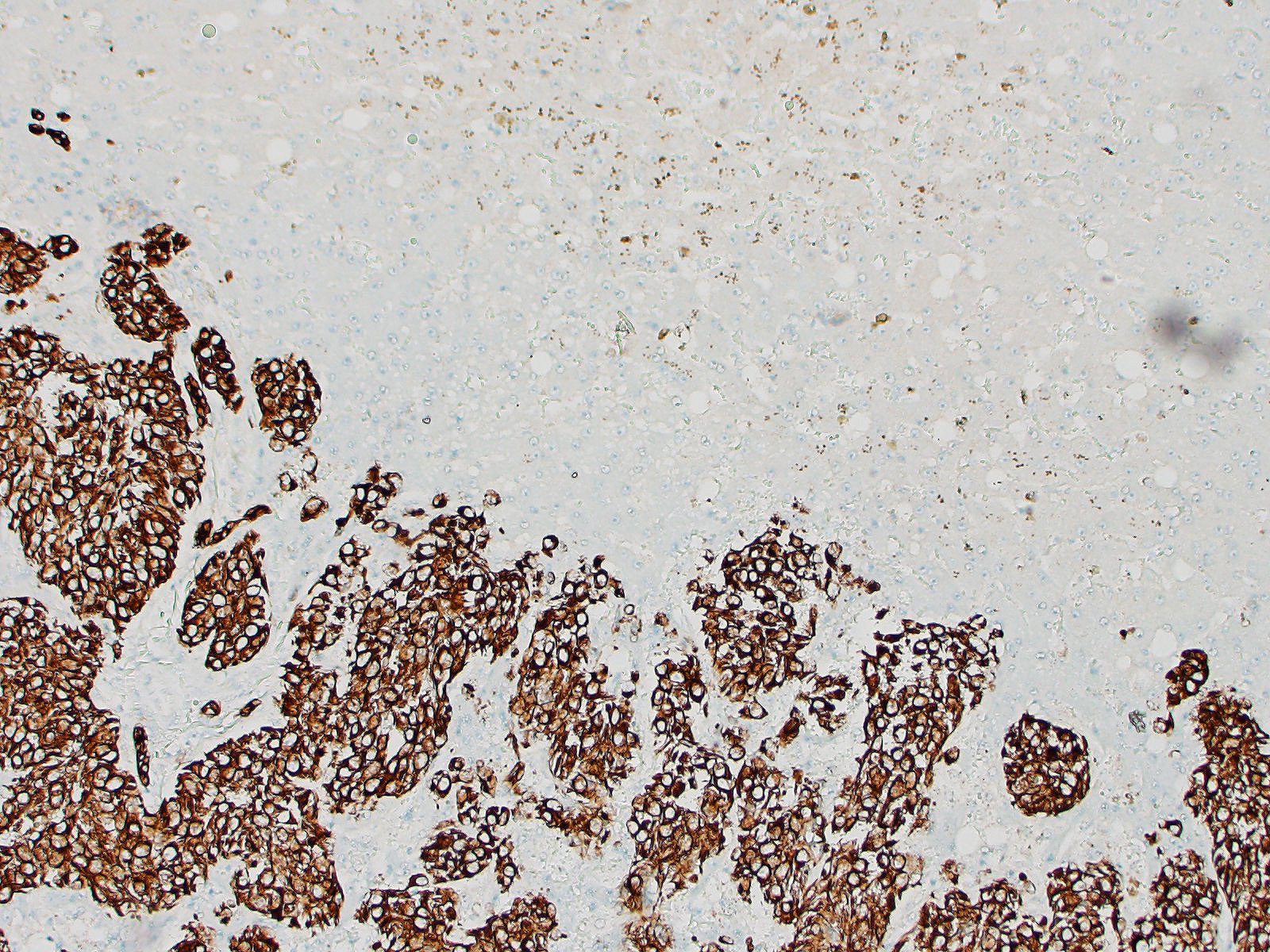
Liver: Multifocally infiltrating and effacing the liver, there is a well demarcated, densely cellular, infiltrative and non-capsulated neoplastic proliferation. It is composed of confluent small islands, ribbons and nests of neoplastic cells showing occasionally central necrotic cores (comedonecrosis) and separated by moderate amount of edematous eosinophilic fibro-vascular tissue (desmoplasia). Neoplastic cells are polygonal, variably sized 15-40 micrometers in length, showing indistinct cell borders, a moderate amount of eosinophilic fibrillary cytoplasm and a single, oval nucleus, bearing vesicular chromatin and 1-2 prominent nucleoli. Anisocytosis and anisokaryosis are moderate and 6 mitoses are observed in 10 HPF (40x objective). Neoplastic islands are also occasionally observed within blood vessels (tumor emboli). Embedded within the fibroblastic stroma, there are hemorrhages and scattered small compressed hepatocytes (compression atrophy). These hepatocytes occasionally have intracytoplasmic finely granular dark brown to green pigment (likely lipofuscin). Centrilobular hepatocytes shows frequently intracytoplasmic lipofuscin (geriatric finding).
Contributor’s Morphologic Diagnoses:
Haired skin– severe bilateral ventrally-oriented symmetrical alopecia with glistening appearance with multifocal erythema and crusts.
Haired Skin – Mild multifocal to diffuse epidermal hyperplasia, with miniaturized and atrophic telogen hair follicles and multifocal parakeratotic hyperkeratosis
Haired skin – mild perivascular to interstitial neutrophilic and mastocytic dermatitis, with dermal edema
Liver – cholangiocarcinoma
Contributor’s Comment:
The present case was characterized by widespread ventrally oriented bilateral and symmetrical alopecia revealing a shiny and glistening skin, in conjunction with multiple hepatic masses, extending to the pancreas. Histological examination revealed a widespread epidermal thickening with smaller hair follicles, containing very rare trichilemmal keratin. There was also a hepatic carcinoma, whose origin is most compatible with a cholangiocarcinoma. Based on the present findings, a diagnosis of feline paraneoplastic alopecia was made.
Feline paraneoplastic alopecia (FPA) is an infrequent but characteristic cutaneous disorder and has been reported in feline patients with a variety of neoplasms. Usually, FPA manifests between 7 and 16 years of age. It is classically associated with pancreatic carcinomas and bile duct carcinomas; nevertheless, other tumors such as hepatocellular carcinoma,4 metastasizing intestinal carcinoma,3 pancreatic neuro-endocrine and hepato-splenic plasma cell tumour1 have been associated with this condition.
The characteristic glistening appearance with the mild epidermal hyperplasia and the severe atrophy and miniaturization of the hair follicles are considered key elements in the diagnosis. The skin is shiny, inelastic but not fragile, in contrast to that in hyperadrenocorticism: the latter is characterized by atrophic and telonigenized hair follicles with epidermal atrophy rather than the hyperplasia observed in this case.
In FPA, it is reported that the stratum corneum is often missing, likely due to excessive grooming (although a clear evidence for this is lacking). This change, together with the patchy parakeratosis, is what is considered to give the spectacular shiny appearance. In the present case, both parakeratosis and absence of stratum corneum are present and alternating one with the other. Although the stratum corneum is often absent, the absence of the granular layer of the epidermis is inconsistently reported in the FPA reports.4
As in other reported FPA cases,6 crusting, in association with Malassezia sp. proliferation was observed in the ear pinna skin but not in the submitted skin sample, and there was also paw pads fissuring and ulceration.
Adnexae are usually normal or mildly atrophic. In the present case, normal sebaceous glands were intermixed with mildly atrophic ones. The apocrine glands, instead, were diffusely dilated and lined by flattened epithelial cells exhibiting evident suprabasilar cleft-like changes, compatible with either a genuine supra-basilar vacuolar degeneration or artefact.
Another unreported and striking finding was the presence of eosinophilic acellular (congo red negative) material in the deep lymphatic vessels, possibly suggestive of a protein rich fluid. There was some discussion about the origin of this change, as they could represent dilated mammary glands/ducts lined by very atrophic epithelial cells.
The hepatic neoplasia was cytologically and behaviorally malignant and consistent with an epithelial origin (i.e. carcinoma). The nodule in the pancreas showed an overlapping morphology with hepatic one, complicating the identification of the origin of the neoplasm. According to the bigger size of the hepatic masses and the histomorphology, a bile duct carcinoma (cholangiocarcinoma) is believed to be more likely. Nevertheless, other tumors, including exocrine pancreatic carcinoma or neuro-endocrine pancreatic carcinoma cannot be fully excluded.
The pathogenesis of this cutaneous lesion has not been understood, due to its relative rarity. Considering that all the FPA-related tumors reported show a variable neoplastic invasion of the liver, it can be hypothesized that some hepatic metabolic derangement are responsible of the cutaneous disorder.
Contributing Institution:
University of Liverpool, Institute of Infection, Veterinary and Ecological Sciences
Department of Veterinary Anatomy, Physiology and Pathology
Leahurst campus
Chester High Road, Neston,
CH64 7TE,
United Kingdom
JPC Diagnosis:
- Liver: Carcinoma, poorly differentiated.
- Haired skin, epidermis and adnexa: Atrophy, diffuse, severe, with telogenization of follicles.
JPC Comment:
The contributor lists two differential diagnoses that were discussed by conference participants: pancreatic carcinoma and neuroendocrine tumor. Participants could not definitively determine the cell of origin based on H&E and thus favored the diagnosis of poorly differentiated carcinoma. Additionally, participants remarked on the hepatic stellate cell (Ito cell) hyperplasia present throughout the liver, which may have been secondary to or unrelated to the neoplasm.
This case is a classic example of paraneoplastic syndrome: the manifestation of a disease in a separate organ system secondary to malignancy elsewhere in the body. While paraneoplastic syndromes in the integumentary system are uncommon in animals, several distinct conditions have been reported.1 Since integumentary lesions are easily detected by owners and often precede signs of the primary malignancy, paraneoplastic syndromes are frequently the reason for initial presentation of the patient.
Exfoliative dermatitis is another example of paraneoplastic syndrome causing alopecia in cats. This syndrome occurs secondary to thymomas in cats, rarely in rabbits, and anecdotally in a dog, though spontaneous (non-neoplastic) cases occur in cats as well. 5, 7 Grossly, there is patchy to diffuse scaling and alopecia, with accumulation of brown waxy material around the claws and mucocutaneous junctions.5 Histologically, there is variable lymphocytic interface dermatitis and mural folliculitis with orthokeratotic hyperkeratosis, hydropic degeneration of keratinocytes, and decreased to absent sebaceous glands. 5, 7
Paraneoplastic pemphigus has rarely been reported in dogs, a cat, and a horse and have occurred secondary to sarcomas and thymic lymphomas.1,5,7 This is a rapidly progressive, severe condition characterized by blistering, erosions, and ulcerations at the mucocutaneous junctions and on mucosal surfaces. Histologically, the syndrome shares features of pemphigus vulgaris, with suprabasilar acantholysis, and erythema multiforme, with a cell-rich lymphohystiocytic interface dermatitis.5, 7
Necrolytic migratory erythema, also known as superficial necrolytic dermatitis or hepatocutaneous syndrome, occurs in dogs and rarely in cats secondary to glucagonoma and certain non-neoplastic conditions like diabetes mellitus and hepatic disease.1, 5, 7 In cats, the condition has also been reported secondary to pancreatic carcinoma, hepatic carcinoid, and lymphoma.7 Grossly, there is scaling and alopecia with erythema, blistering, and ulceration around the face, pinnae, and pressure points (i.e. elbows). The paw pads are frequently thickened and fissured.1, 5, 7 Histologically, there is diffuse parakeratotic hyperkeratosis, severe hydropic degeneration and acanthosis within the superficial stratum spinosum, and hyperplasia in the deeper stratum spinosum and stratum basale, imparting a distinct red, white, and blue striped appearance, and there is minimal inflammation. 5, 7
Nodular dermatofibrosis is another paraneoplastic syndrome of the skin which occurs in German shepherd dogs and occasionally other large breed dogs in conjunction with renal cysts, renal cystadenocarcinomas, and uterine leiomyomas.1, 5 These manifest as multifocal, slow-growing, nodular collagenous hamartomas typically in the subcutis and dermis of the extremities.1, 5 Other reported paraneoplastic syndromes affecting the skin include necrotizing panniculitis (secondary to pancreatic neoplasia or pancreatitis in dogs, cats, and horses), cutaneous flushing (secondary to pheochromocytomas in dogs), and generalized pruritus (secondary to lymphoma in dogs and horses).1, 5
References:
- Bergman PJ. Paraneoplastic Syndromes. In: Withrow SJ, Vail DM, Page RL, eds: Withrow and MacEwen’s Small Animal Clinical Oncology. 5th St. Louis, MO: Elsevier Saunders; 2013: 89-90.
- Caporali C, Albanese F, Binanti D, Abramo F. Two cases of feline paraneoplastic alopecia associated with a neuroendocrine pancreatic neoplasia and a hepatosplenic plasma cell tumour. Vet Dermatol. 2016;27:508-e137
- Grandt LM, Roethig A, Schroeder S, et al. Feline paraneoplastic alopecia associated with metastasising intestinal carcinoma. JFMS Open Rep. 2015:2055116915621582.
- Marconato L, Albanese F, Viacava P, Marchetti V, Abramo F. Paraneoplastic alopecia associated with hepatocellular carcinoma in a cat. Vet Dermatol. 2007 ;18:267-71.
- Mauldin EA, Peters-Kennedy J. Integumentary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 1. St. Louis, MO: Elsevier. 2016; 586, 603, 691-693.
- Turek MM. Cutaneous paraneoplastic syndromes in dogs and cats: a review of the literature. Vet Dermatol. 2003;14:279-96.
- Welle MM, Linder KE. The Integument. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th St. Louis, MO: Elsevier. 2022: 1209, 1255, 1261.