Signalment:
Gross Description:
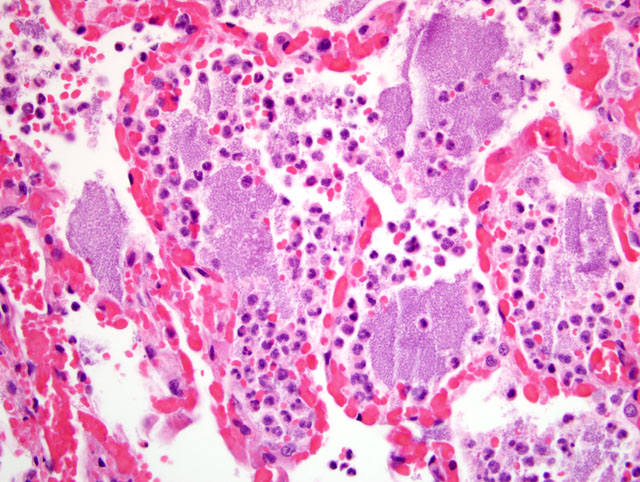
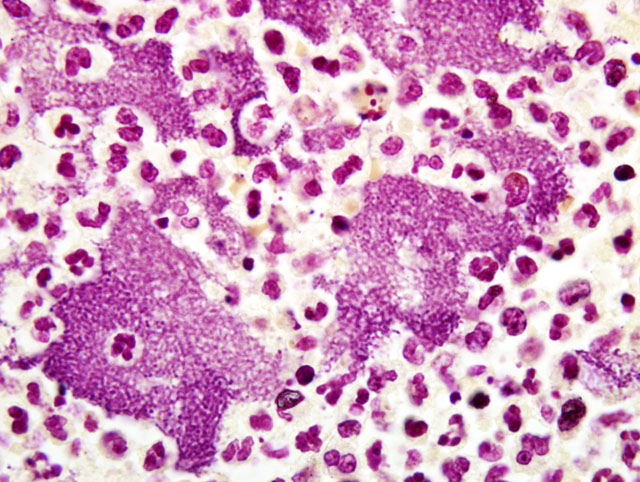
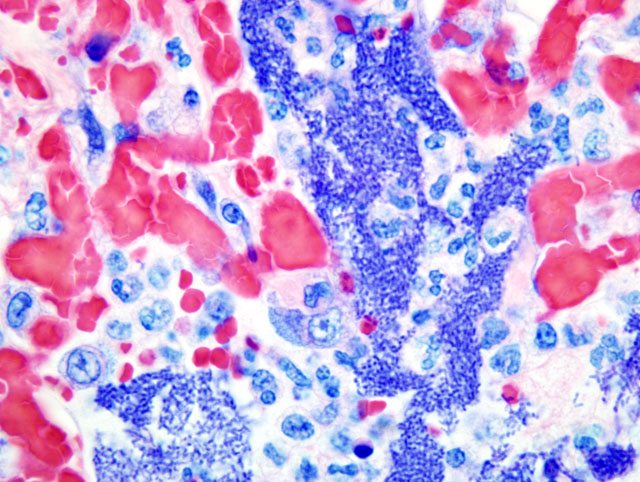
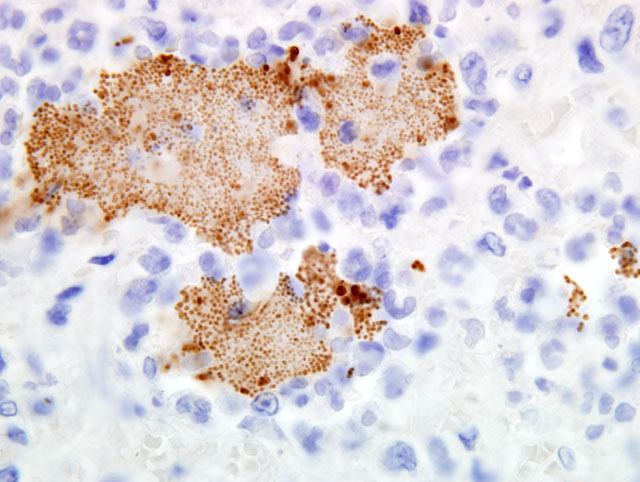
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Cats (and dogs) acquire the disease most commonly following predation on infected rodents and lagomorphs or by bites from the preys plague-infected fleas. The season for plague in New Mexico begins in mid-May, and peaks in August, tailing off in October. However, cases occur at any time with proper conditions. In New Mexico, plague cases increase after unusually wet winter and spring weather; these conditions are conducive to increase in the rodent (and subsequently, flea) population.
The lesions seen in Y. pestis-infected animals vary according to the mode of transmission of the organism and susceptibility of the host. The three classic clinical manifestations of Y. pestis infection include bubonic, pneumonic and septicemic plague and are seen primarily in susceptible non-rodent species. Cats submitted to our laboratory typically have some type of enlargement of tonsils, cervical lymph nodes, or both. These may or may not present as draining lesions. Exudate produced is typically mucoid, grey, and contains large numbers of bacteria. When the disease has progressed to the lungs, all lobes of both lungs will typically be deep reddish-purple, very congested, and often will sink (or just barely float) in formalin. When presented with a suspect plague case (either clinically or in the laboratory), it is important to take proper precautions with the animal. Intense and immediate flea control is paramount with these cases; in our laboratory, suspect cases are routinely sprayed liberally with an appropriate pyrethrin based insecticide prior to any examination or handling.
Clinically, these animals usually present with some type of oral lesion, salivating, gagging, respiratory difficulty or distress, and lymph node enlargement accompanied by a high temperature. There may be a draining lesion from the cervical lymph node region or tonsils. When diagnosed early, these animals respond readily to appropriate antibiotic therapy. The disease is so common in Santa Fe County in the summer months as to commonly be one of the differentials in ADR (i.e. aint doing right) cats. This disease is of importance as a public health risk, and as one of the select agents of bioterrorism.
JPC Diagnosis:
Conference Comment:
The virulence of Yersinia sp. depends on its ability to invade the host and evade host immune responses. This begins in the gut of the infected flea, where Y. pestis forms a biofilm that obstructs the gut; the flea then must regurgitate before feeding, and thus infects the host that it bites. Via an elaborate gene complex, the Yop virulon, Yersinia spp. form proteins that assemble into a type III secretion system, a hollow tube that projects from the bacterial surface, binds to host cells, and injects the bacterial toxins, known as Yersinia outercoat proteins (Yops). YopE, YopH, and YopT interfere with actin polymerization inside the host cell, thus blocking phagocytosis of the bacterium. YopJ inhibits the production of inflammatory cytokines by inhibiting the signaling pathways that are activated by lipopolysaccharide.(9)
While natural Y. pestis infections still occur in humans, large outbreaks have been prevented by improved sanitation and prompt, successful antibiotic treatment of patients with the bubonic form of the disease. However, it is now apparent that that Y. pestis can acquire multidrug resistance via plasmids that are readily horizontally transmitted, and in 1995, two fatal cases of bubonic plague were caused by multidrug resistant Y. pestis. As a result, the threat of Y. pestis emergence as a deadly, multidrug resistant epidemic has prompted renewed interest in vaccination as a best line of defense against resurgent plague.(1)
Because whole cell vaccines are considered potentially unsafe and unreliable, vaccine research has focused on two antigens, F1 and LcrV. The F1 protein is a bacterial surface antigen that aids Y. pestis in evading recognition by the innate immune system; it is not essential for virulence, however, and is therefore not considered sufficient as a sole vaccine antigen. The LcrV protein is essential for virulence, and mediates insertion of a translocation pore, assembled via the type III secretion system, in the host cell membrane. Vaccines which combine both LcrV and F1 show promise against bubonic and pneumonic plague in animal models. The Brown Norway rat has emerged as a model for both human bubonic and pneumonic plague.(1)
References:
2. Behr M: Clarification about plague and its diagnosis. J Am Vet Med Assoc 211(6):698, 1997
3. Cleri DJ, Vernaleo JR, Lombardi LJ, Rabbat MS, Mathew A, Marton R, Reyelt MC: Plague pneumonia disease caused by Yersinia pestis. Semin Respir Infect 12(1):12-23, 1997
4. Eidson M, Thilsted JP, Rollag OJ: Clinical, clinicopathologic and pathologic features of plague in cats: 119 cases (1977-1988). J Amer Vet Med Assoc 199:1191-1196, 1991
5. Ettestad, PE: Veterinary Epidemiologist, NM Dept Health, Personal Communication, July, 2005
6. Lappin MR: Feline zoonotic diseases. Vet Clin North Am Small Anim Pract 23(1):57-78,1993
7. Lopez T: Plague. Finding ways to stop a killer. J Am Vet Med Assoc 211(3):280-281, 1997
8. Macy DW: Plague. In: Infectious Diseases of the Dog and Cat, ed. Greene CE, 2nd ed., pp. 295-300. WB Saunders Co., Philadelphia, PA, 1998
9. McAdam AJ, Sharpe AH: Infectious diseases. In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas AK, Fausto N, Aster JC, 8th ed., p. 365. Saunders Elsevier, Philadelphia, PA, 2010
10. Rosser WW: Bubonic plague. J Am Vet Med Assoc 191(4):406-9, 1987
11. Watson RP, Blanchard TW, Mense MG, Gasper PW: Histopathology of experimental plague in cats. Vet Pathol 38:165-172, 2001