Signalment:
Gross Description:
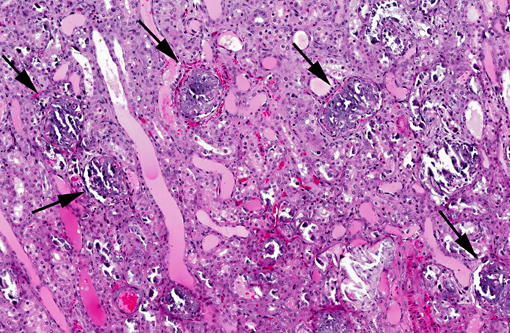
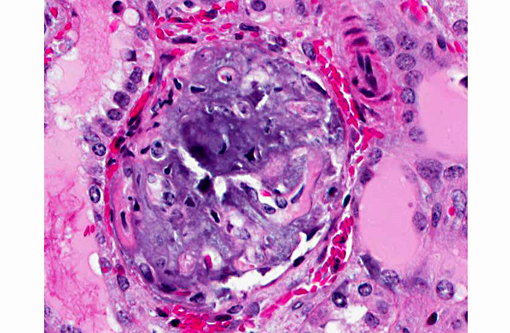
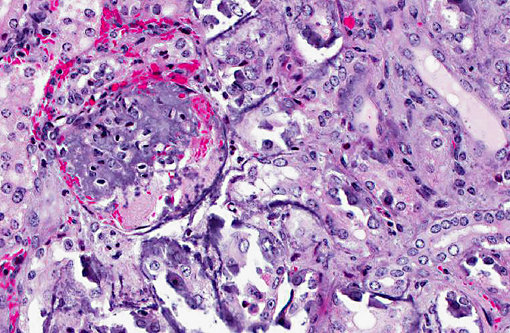
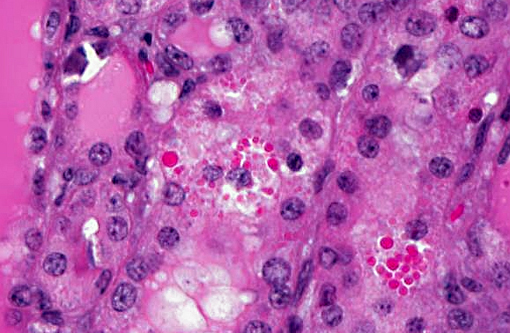
Histopathologic Description:
Additional mineralized areas were found in elastic arteries near the heart, trachea, compartment 3, spleen and adrenal.
Morphologic Diagnosis:
Lab Results:
| Test | 6 days of age | 7 days of age | Normal values in llamas (Merck Veterinary Manual) |
| Glucose | 160 | ND | 90-140 mg/dL |
| Urea nitrogen | 180 mmol/L | 183 | 13-32 mg/dL |
| Creatine | 10.5 | 10.8 | 1.5-2.9 mg/dL |
| Sodium | 147 | 149 | 134-150 mmol/L |
| Potassium | 8.5 | 9.0 | 4.3-5.6 mEq/L |
| Chloride | 110 | 112 | 106-118mEq/L |
| Albumin | 1.7 | 2.0 | 2.6-4.7 mg/dL |
| Total protein | 3.3 | ND | 5.6-7.3 mg/dL |
| Calcium | 13.1 | 13.1 | 2.0-2.6 |
| Phosphorus | 13.4 | 14.6 | 4.4-8.5 |
| AST | 141 | ND | 10-280 IU/L |
| GGT | 17 | ND | 5-29 U/L |
| CK | 65 | ND | 10-200 IU/L |
Condition:
Contributor Comment:
Rickets is considered a common clinical problem in camelids and is particularly frequent in crias born in September to March period particularly in the high latitudes of the northern and southern hemispheres. Prolonged inclement weather and conditions of reduced sunlight can be contributory to vitamin D deficiency under these conditions.(4) This animal was born in late August. Thus many owners supplement.
This cria was given a goat colostrum supplement fortified with vitamin D due to the owners concern that rickets was a possibility.(3) This supplement has been reported as a cause of hypervitaminosis D in alpaca crias previously. Among the signs found in other over-supplemented goats are hypercalcemia, hyperphosphatemia and renal dysfunction as seen in this case.
Metastatic calcification occurs in otherwise normal tissue due to hypercalcemia secondary to some disturbance in calcium metabolism. Entry of large amounts of calcium into cells results in its precipitation in organelles. Common causes are renal, vitamin D intoxication [commonly affects aorta, atrial and left ventricular endocarcium, lungs], elevated PTH or PTH-related protein, and neoplastic destruction of bone.(7) Common target tissues are gastric mucosa, kidney, lung, systemic arteries and pulmonary veins. Many of these cells lose acid and therefore have an alkaline internal compartment predisposing to deposition of mineral salts.
Calciferol and D3 localize in nucleus as do other steroids, turning on genes for increased calcium transport. In addition to being found in young animals due to vitamin overdose, as in this instance, pets ingesting Rampage rodent poison have similar lesions.
JPC Diagnosis:
Conference Comment:
Common causes of hypercalcemia include hyperparathyroidism, hypoadrenocorticism, acidosis, renal disease (in horses and some dog breeds), vitamin D toxicity, prolonged immobilization, osteolytic lesions, neoplasia (lymphoma, canine adenocarcinoma of the anal sac apocrine glands, plasma cell myeloma, some carcinomas), thiazide diuretics and granulomatous inflammation. Hyperproteinemia and hemoconcentration will also falsely elevate serum calcium.(2) Common causes of hyperphosphatemia include hemolysis, nutritional 2o hyperparathyroidism, hyperthyroidism, hypervitaminosis D, osteolytic bone lesions, hypoadrenocorticism, renal failure (in most species except for horses), hypoparathyroidism, tumor lysis and administration of phosphate-containing fluids or enemas. Relatively high phosphorus is normal in young animals.(2) In this case, serum chemistry revealed both hypercalcemia and hyperphosphatemia, narrowing the differential diagnosis down to hypervitaminosis D, osteolysis or hypoadrenocorticism. The history of repeated vitamin D supplementation supports a diagnosis of renal mineralization secondary to vitamin D toxicity. The marked azotemia is secondary to renal failure, while hyperkalemia is attributed to renal failure or acidosis.
Although excessive dietary supplementation of vitamin D is the most frequent cause, ingestion of cholecalciferol-containing rodenticides or plants containing vitamin D glycosides (Cestrum dirunum, Solanum malacoxylon, Trisetum flavescens and Medicago saliva) have also been implicated in cases of vitamin D toxicity.(5) Vitamin D maintains plasma levels of calcium and phosphorus by acting on the small intestine, bone, and kidneys. Specifically, it promotes active uptake and transcellular transport of calcium by increasing calbindin synthesis; it stimulates renal calcium absorption in distal tubules; and it stimulates mobilization of calcium and phosphorus from bones. The latter occurs upon binding of osteoblast RANKL (receptor activator for NF-kB ligand) to preosteoclast RANK, which induces differentiation into mature osteoclasts and initiates bone resorption via secretion of HCl and proteases such as cathepsin K. Additionally, vitamin D contributes directly to mineralization of epiphyseal cartilage and osteoid matrix by stimulating osteoblasts to synthesize the calcium-binding protein osteocalcin, which is involved in mineralization of these matrices.(1)
Vitamin D is obtained directly from dietary sources or synthesized endogenously from a precursor (7-dehydrocholesterol) that is present in the skin. Irradiation of 7-dehydrocholesterol with ultraviolet light induces the formation of cholecalciferol (vitamin D3). The precursor in plants is ergosterol, which is converted to vitamin D2 by ultraviolet light and then converted to vitamin D3 in the body.(1) Inactive cholecalciferol (vitamin D3) binds to plasma α1-globulin and is transported to the liver. There, it is converted by hepatic 25-hydroxylases to 25-hydroxy-cholecalciferol (25-OH-D). Finally, renal α1-hydroxylase converts 25-OH-D to active 1,25-dihydroxycholecalciferol. Regulation of renal vitamin D production occurs through three major mechanisms. Hypocalcemia upregulates parathyroid hormone production, which induces activation of α1-hydroxylase and thus increases 1,25-dihydroxycholecalciferol production. Hypophosphatemia directly activates of α1-hydroxylase, resulting in a similar increase in 1,25-dihydroxycholecalciferol production. Conversely, increased levels of 1,25-dihydroxycholecalciferol provoke negative feedback inhibition of α1-hydroxylase.(1) New world monkeys are entirely dependent upon dietary sources of vitamin D since it cannot be synthesized in their skin and, as noted by the contributor, vitamin D availability is considered low in camelids, a condition which is exacerbated during the winter at high latitudes when ultraviolet radiation is decreased.(1)
References:
2. Ferguson DC, Hoenig M. Endocrine system. In: Latimer KS, ed. Duncan and Prasses Veterinary Laboratory Clinical Pathology. 5th ed. Ames, IA: Wiley Blackwell; 2011:295-304.
3. Gerspach C, Bateman S, Sherding R, et al. Acute renal failure and anuria associated with vitamin D intoxication in two alpaca (Vicugna paco) cria. J Vet Intern Med. 2010;24:423-429.
4. McClanahan SL, Wilson JH, Anderson KL. What is your diagnosis? J Amer Vet Med Assoc. 2006; 229(4):499-500.
5. Thompson K. Bones and joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals, 5th ed. Vol. 1. St. Louis, MO: Elsevier Limited; 2007:10-11, 58-59.
6. Van Suan RJ. Nutritional diseases of llamas and alpacas. Vet Clin North Am Food Anim Pract. 2009;25(3):797-810.
7. Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis MO: Elsevier Mosby; 2012:40.