Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 12
11 December 2019
CASE II: A12-357 (JPC 4031109).
Signalment: 4 year old male rhesus macaque (Macaca mulatta)
History: This rhesus macaque was inoculated with SIVsmE6601:1 as part of a research study. Six months post inoculation, the animal was noted to have poor appetite and acute weight loss (18% body weight over 2 weeks). Clinical examination two days prior to euthanasia revealed severe respiratory distress with nasal discharge and wheezing, along with liquid diarrhea and a severe neutrophilic leukocytosis. Due to poor prognosis and progression towards simian acquired immunodeficiency syndrome, the animal was humanely euthanized.
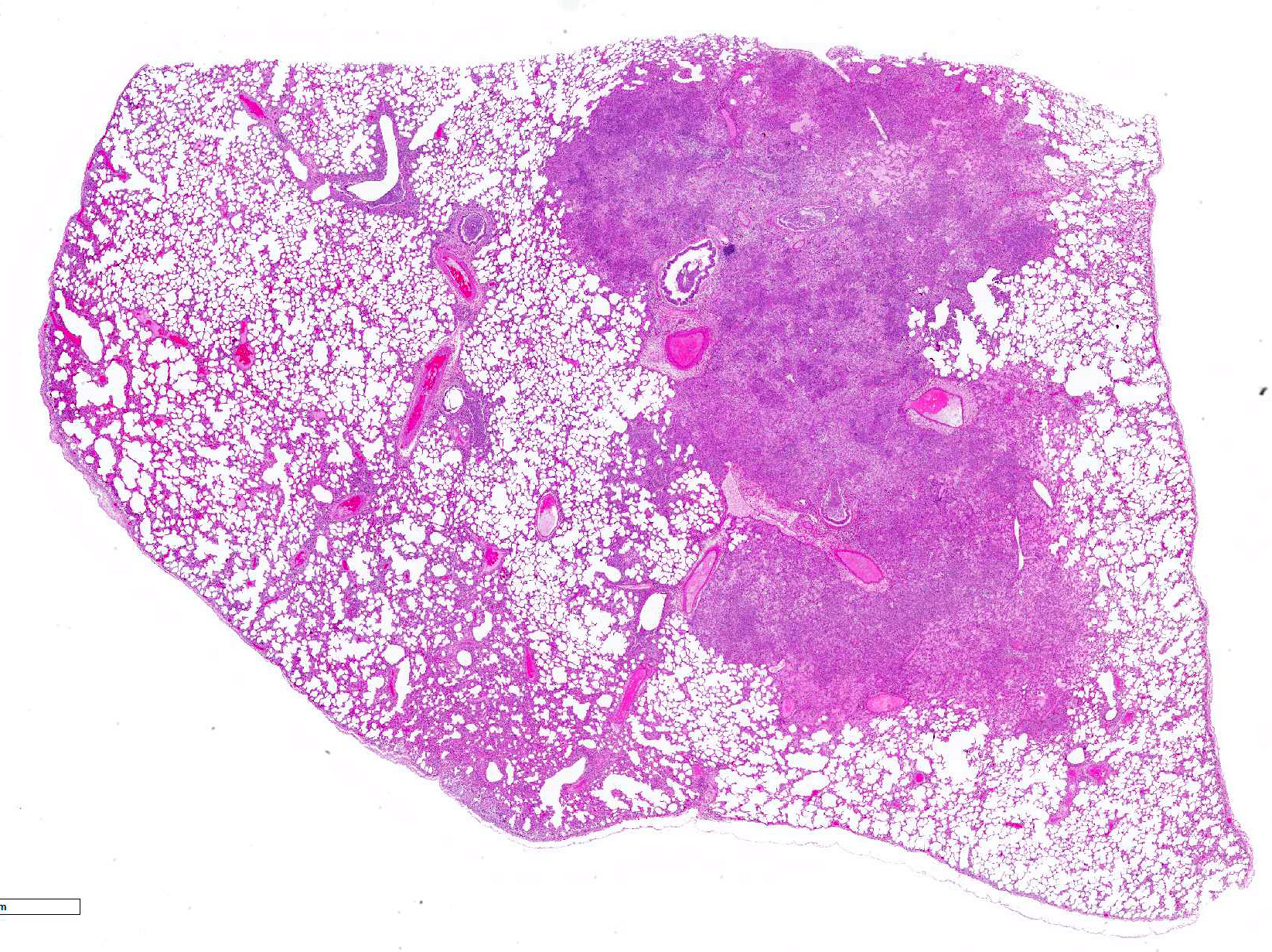
Gross Pathology: The animal was in poor body condition with no appreciable body fat. The mucosa of the stomach was thickened and edematous. The duodenum was diffusely hyperemic. The liver and kidneys had pale foci scattered throughout the parenchyma extending through cut sections. All lung lobes were diffusely reddened and severely mottled dark red or pale brown and markedly thickened and firm.
Laboratory results: N/A
Microscopic Description:
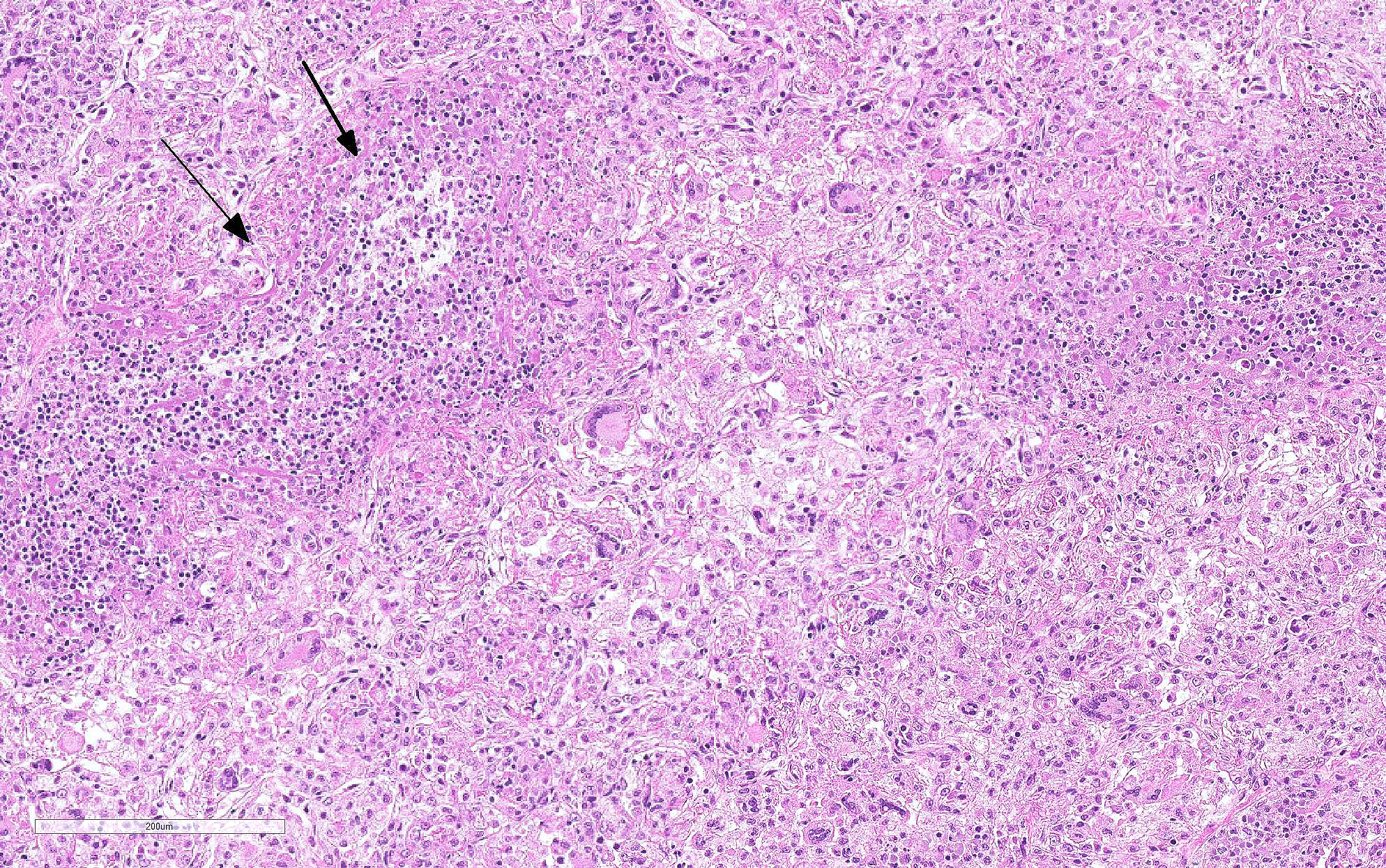
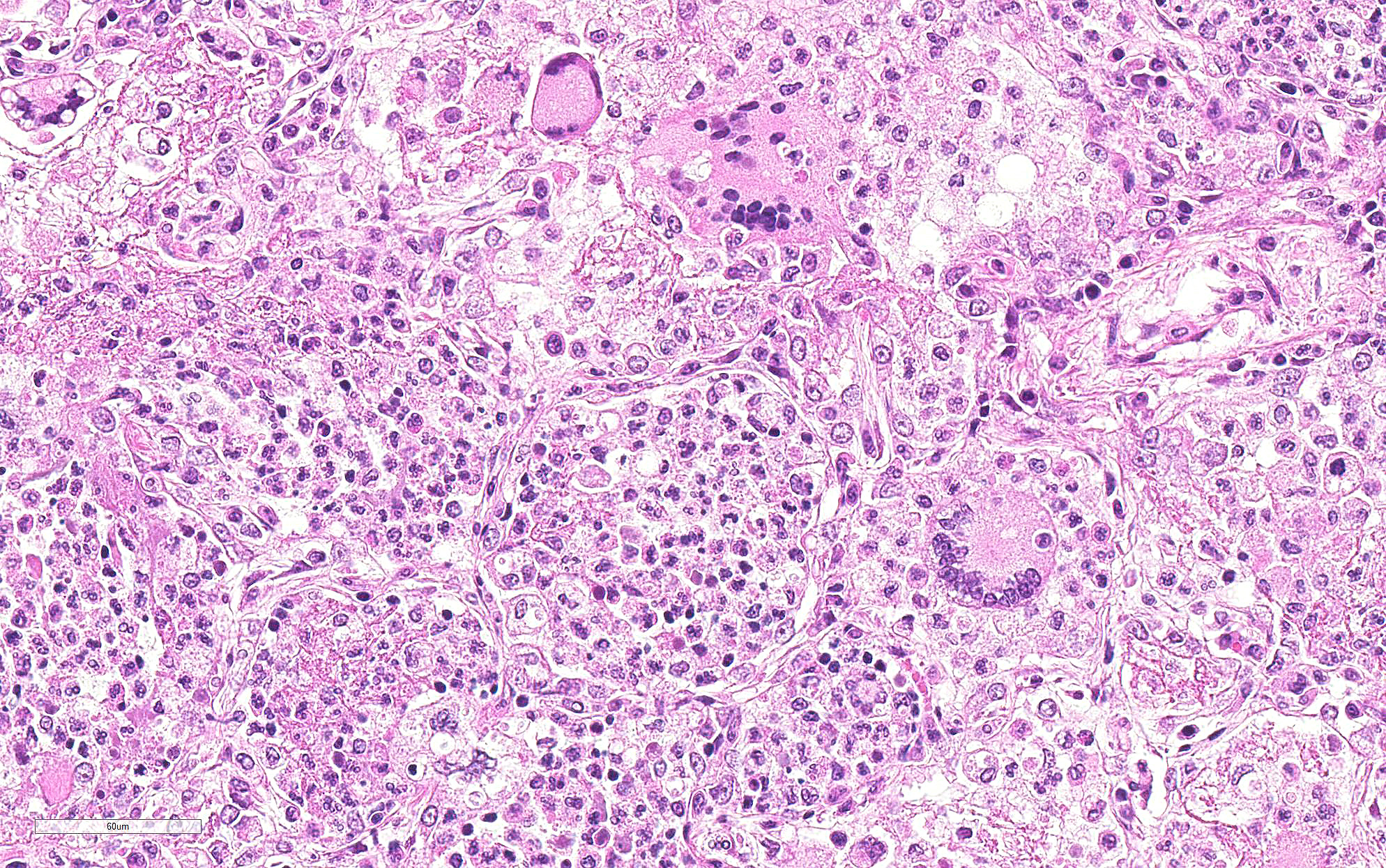
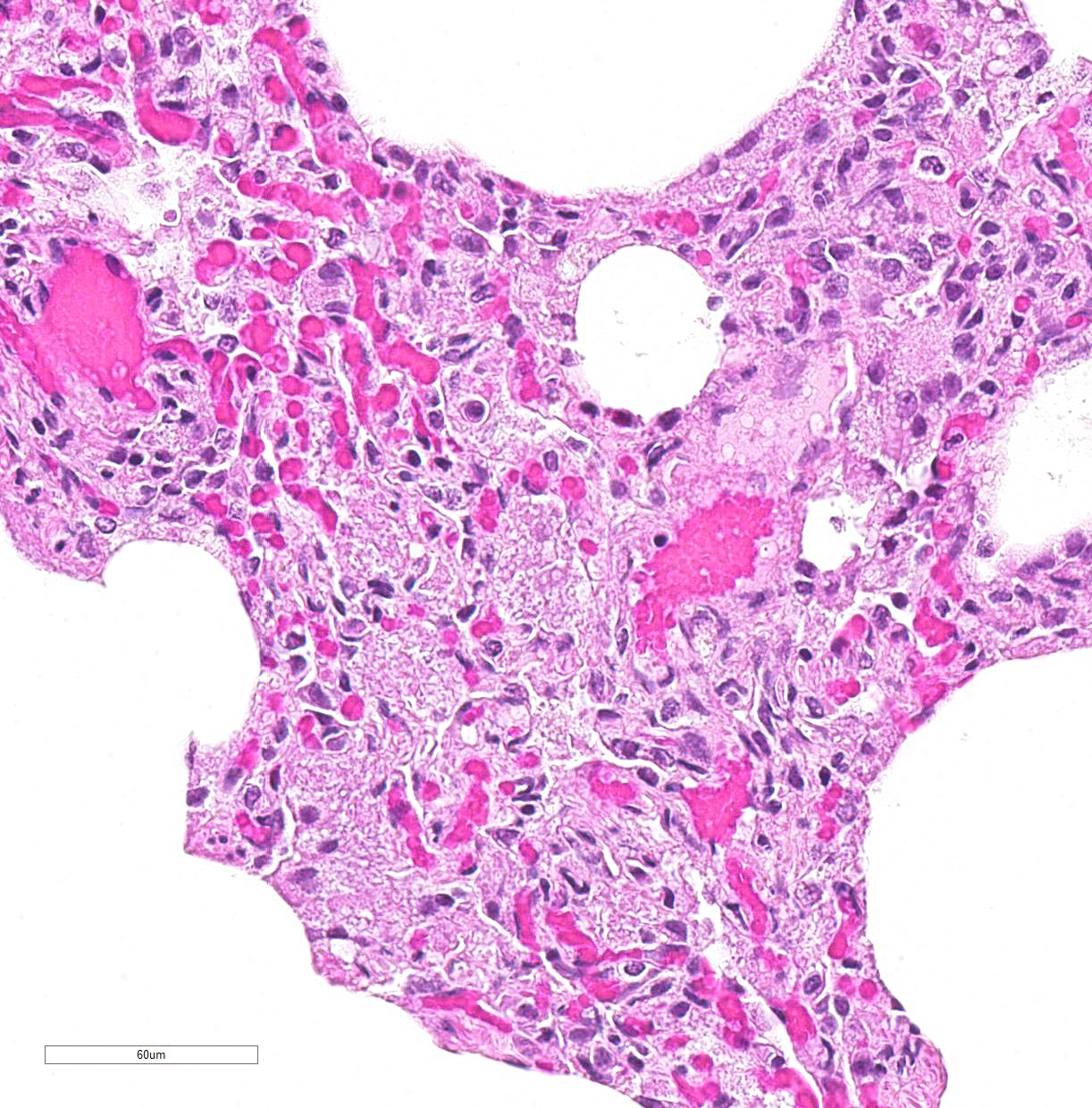
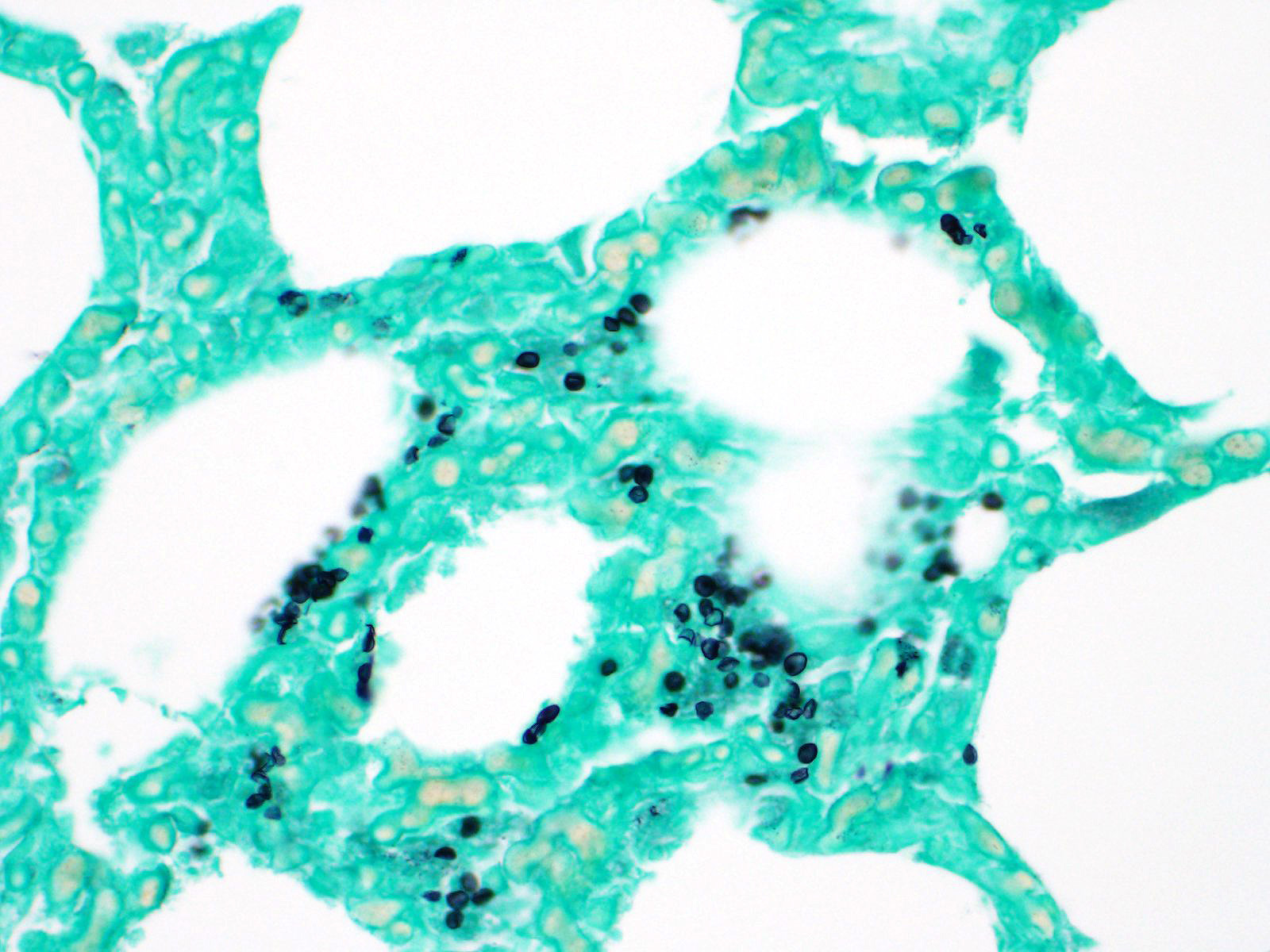
Lung: Primarily focused around bronchi and bronchioles and involving approximately 40% of the tissue examined, alveolar spaces are filled with inflammatory cells consisting of neutrophils, epithelioid macrophages, and multinucleated giant cells admixed with edema, fibrin, cellular and karyorrhectic debris, and extravasated red blood cells. Within several areas of inflammation are bacterial colonies (not present on each slide). The lumina of several bronchioles are partially occluded by neutrophils, histiocytes, cellular and karyorrhectic debris, and fibrin. Multifocally within alveolar spaces, are aggregations of foamy, eosinophilic fungal organisms (Pneumocystis carinii).
Contributor Morphologic Diagnosis:
Lung: Multifocal, severe, chronic bronchointerstitial pneumonia with SIV giant cells and Pneumocystis carinii
Contributor Comment Lentiviruses are a subfamily of retroviruses and include human immunodeficiency virus (HIV), simian immunodeficiency virus (SIV), equine infectious anemia virus (EIAV), ovine lentivirus (OvLV), bovine immunodeficiency virus, and feline immunodeficiency virus (FIV). Lentiviruses cause chronic disease syndromes including immunodeficiency, encephalitis, pneumonia, arthritis, anemia, thrombocytopenia, and lymphoid hyperplasia. SIV has proven a useful model to study HIV pathogenesis, as the virus is morphologically identical to HIV by electron microscopy, serologically related to HIV, and cytopathic for CD4+ T cells.7 SIV infected macaques develop peripheral lymphadenopathy, splenomegaly, diarrhea, weight loss, skin rash, pneumonia, septicemia, and hemogram abnormalities including leukopenia, lymphopenia, neutropenia, anemia, and thrombocytopenia. The appearance of multinucleate giant cells is often an indication of terminal phase of disease progression.7
The most common pulmonary lesion in HIV is a lymphocytic and histiocytic pneumonia, centered around vessels and bronchioles.6 Interestingly, when this syndrome occurs, HIV- infected individuals have lower incidence of opportunistic infections and longer survival times. SIV is similar, and macrophage-tropic strains cause pneumonia1,2,6. If these infected macrophages go on to form giant cells, this is generally the terminal phase of SAIDS. If it infects macrophages and there is a T cell response in the lung, humans with the disease get pneumonia, but not OIs and death.
Pulmonary opportunistic infections in both AIDS patients and SAIDS-infected monkeys include Pneumocystis carinii, Mycobacterium avium-intracellulare, cytomegalovirus, Cryptosporidium parvum as well as other bacteria, fungal, and viral organisms. Pulmonary conditions reported to occur secondary to AIDS or SAIDS include Kaposiâs sarcoma, nonspecific pneumonitis, pulmonary lymphoma, pulmonary lymphoid hyperplasia, and lymphocytic interstitial pneumonia. Pneumocystis carinii infection in macaques is characterized by foamy pink exudate within alveoli, extensive lymphocytic infiltration, and type II pneumocyte hyperplasia.3
Pulmonary multinucleate giant cells and histiocytic infiltrates have been observed in SIV infected rhesus macaques.7 Viral antigen (p26 and p14) has been observed on theses syncytial cells and smaller macrophages in alveolar spaces, circulating in pulmonary blood vessels, and in the sinusoids of tracheobronchial lymph nodes of moribund animals.2 These cells have also been labeled with a-1-antichymotrypsin, indicating macrophage origin. This spectrum of lesions is seen in monkeys that are free from CMV.2 The more common pulmonary change in SIV infection is perivascular mononuclear inflammation between 2-8 weeks after inoculation. SIV arrives at the lung hematogenously likely within lymphocytes or macrophages. This characteristic infiltrate of multinucleate giant cells reflects the final stage of disease where the immune system is unable to restrict viral replication in macrophages. Disseminated giant cell disease is a characteristic of terminal AIDS/SAIDS.3,8
Contributing Institution:
Harvard Medical School
Division of Comparative Pathology
New England Primate Research Center
One Pine Hill Drive
Southborough, MA 01772
www.hms.harvard.edu/NEPRC
JPC Diagnosis: Lung: Pneumonia, interstitial, histiocytic, with abundant fungal cysts, neutrophils, and numerous viral syncytia.
JPC Comment: The contributor provides a concise overview of pulmonary SIV infection in the macaque, which very closely approximates common events as seen in HIV infected humans.
The fungus Pneumocystis carinii was first identified in post-World-War II Europe in malnourished children, and prior to the onset of the HIV epidemic in the 1980s, was most often seen in patients undergoing chemotherapy. Infection elicits humoral and cell mediated responses in affected individuals with the CD4+T-cell-driven response considered the most important in combating infection. Asci, or cystic forms, have beta-glucan within their cell wall, which is recognized by alveolar macrophages and epithelial which ultimately prime the T-cell response. Various inflammatory responses, including Th1, Th2, and Th17 responses all been identified in various cases of PCP, however, none have been shown to be intrinsic to disease clearance. Trophozoites, which lack a cell wall, do not stimulate the response, and may actively inhibit it. Macrophages also appear to be the primary cell responsible for killing the fungus.
Coinfection by Pneumocystis carinii in SIV-infected macaques with AIDS is a common finding, especially in terminal stages of the disease. Experimental models of Pneumocystis carinii pneumonia (PCP) infection in rhesus macaques have been established via pulmonary airway inoculation of SIV-infected animals, which allows the ability to study the pathogenesis of PCP in immunosuppressed patients. Characteristic findings in this model parallels those seen in natural infection, with an initial increase in CD8+ T cells in the lung and bronchiolo-alveolar lavage fluid of affected animals until they constitute over 90% of CD3+ T-cells. Infiltration of neutrophils into the lung generally heralds the onset of clinical disease and are considered a major cause of pulmonary damage in PCP.
It was generally accepted for many years among SIV researchers that the African nonhuman primates, which infected with lentiviruses do not progress to AIDS (as characterized by CD4+ depletion, opportunistic infections, and neoplasia). A 2009 paper by Pandrea et al8 chronicled progressive SIV infection in African green monkeys, mandrills, sooty and black mangabeys, and HIV infection in baboons and chimpanzees. While the viral loads required to infect these species is considerably higher than required to infect Asian macaques, and potentially higher than natural infection, it may be wise to consider same- and cross-species infection with lentivirus a "persistently non-progressive" rather than "non-pathogenic" infection in these species.8
References:
4. Board KF, Patil S, Lebedeva I, CapuanoIII S, Trichel AM, Murphey-Corb M, Rajakumar PA, Flynn JL, Haidaris CG, Noris KA. Experimental Penumocystic carinii pneumonia in simian immunodeficiency virus-infected rhesus macaques. J Inf Dis 2003; 187:576-588.
5. Hoving JC, Kolls JK. New advances in understanding the host immune response to Pneumocystis. Curr Opin Microbiol 2017; 40:65-71