WSC 22-23
Conference 7
CASE III:
Signalment:
67-day old, male, Holstein, bovine (Bos taurus)
History:
The calf was transferred to a farm for fattening at 7-days old. The calf did not receive any vaccinations and developed respiratory signs, including coughing; the condition worsened despite antibacterial treatment with oxytetracycline. The calf was euthanized at the age of 67-day old due to anorexia and respiratory distress.
Gross Pathology:
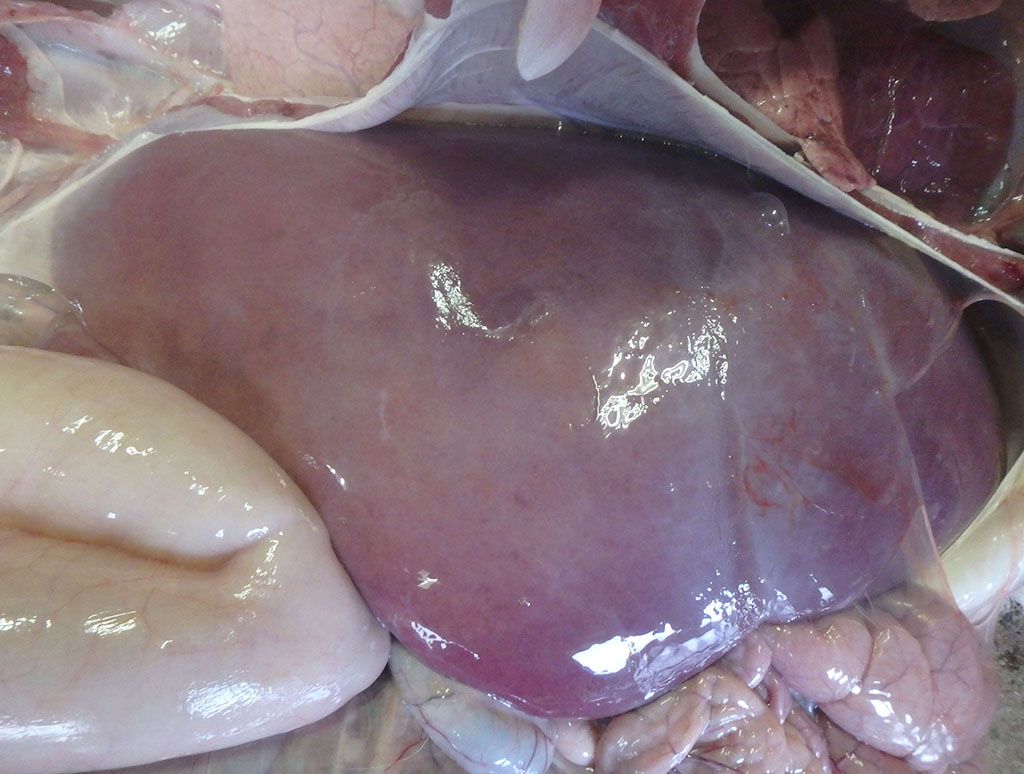
At necropsy, the liver was swollen and faded in color, with multiple, micro-yellow-white foci on the cut surface. The lungs showed hepatization in the anterior and middle lobes and regression failure in the posterior lobes. Multiple petechiae and white foci were detected in the renal cortex. The spleen was congested and swollen. The growth of rumen papillae was poor, and the contents were muddy with an acidic odor. The pulmonary hilum, hepatic hilum, and mesenteric lymph nodes were edematous and swollen.
Laboratory Results:
Bacterial colonies morphologically and biochemically consistent with Salmonella enterica were isolated from the liver, spleen, kidney, lung, pulmonary hilar, and mesenteric lymph nodes by direct culture, and formed
the whole blood, rumen, and cecal contents in an enriched culture. The isolates were identified as S. enterica subsp. enterica by 16S rDNA sequencing and serotyped as Salmonella Dublin (serotype O9: g, p :-). The isolates contained the invA and spvC genes. No virological examination was performed.
Microscopic Description:
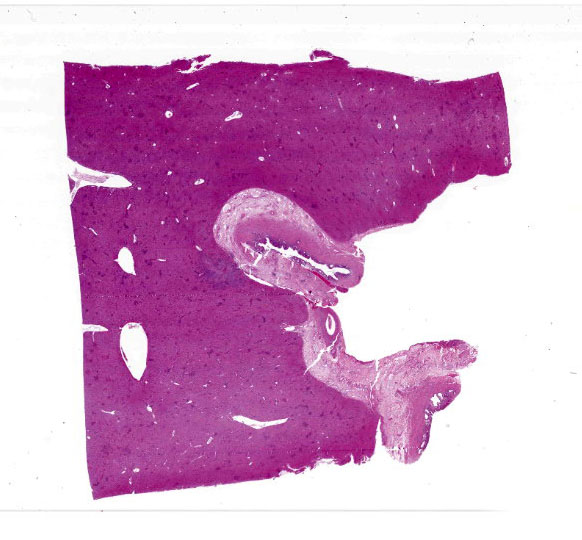
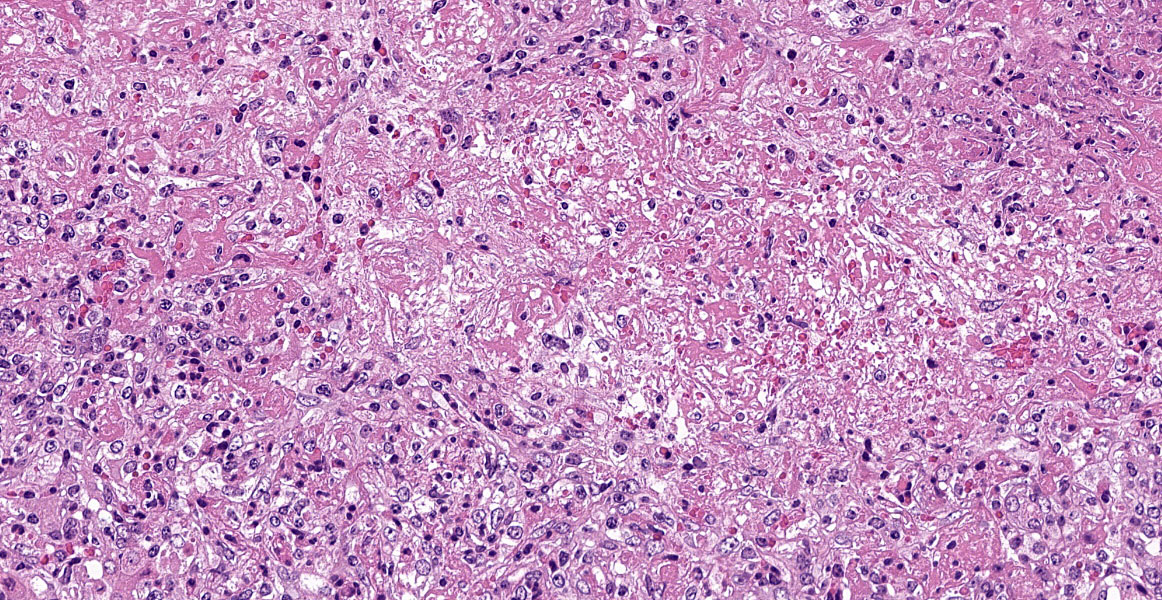
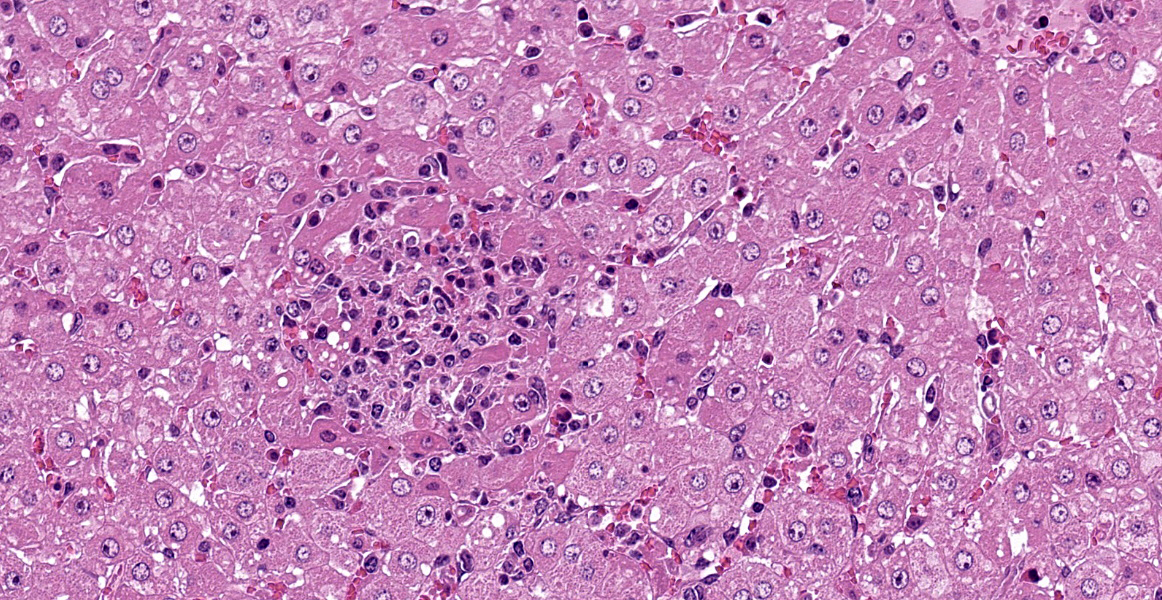
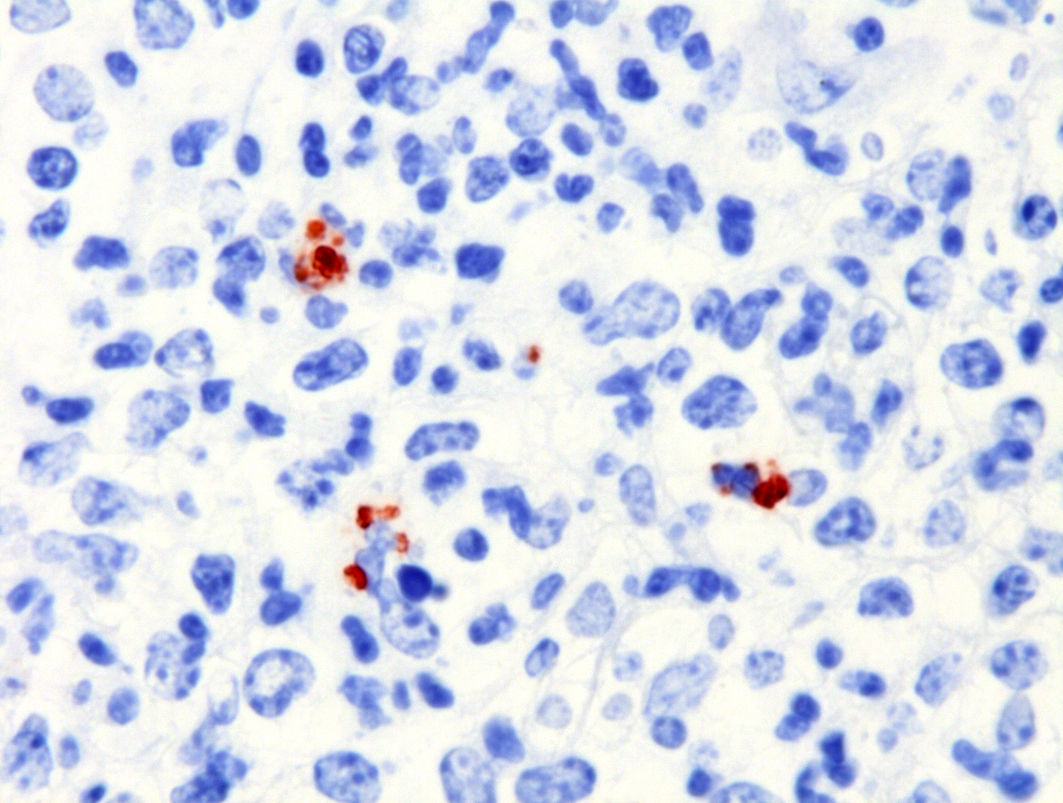
Severe multifocal hepatic necrosis and paratyphoid nodules were observed and characterized by histiocytic, lymphocytic, and neutrophil infiltration. Infiltration of lymphocytes and plasma cells was also detected in Glisson’s capsule. A hyaline thrombus was observed in the central vein. In the kidney, severe multifocal pyogranulomatous interstitial nephritis was detected with hemorrhage. Similar lesions were found in the spleen and lung interstitium, as well as in the submucosa of the rumen, ileum, and cecum. Immunostaining for anti-Salmonella O9 rabbit serum showed positivity in the cytoplasm of macrophages in the hepatic lesions.
Contributor’s Morphologic Diagnoses:
Liver: hepatitis, multifocal, necrotizing, histiocytic, lymphocytic and neutrophilic, paratyphoid nodules, Salmonella enterica subsp. enterica Dublin, Bos taurus, bovine
Contributor’s Comment:
Salmonellosis is a zoonotic, enteric, or multisystemic disease, distributed worldwide.1, 3, 6,7,9-11 This infection causes huge economic losses to the food animal industry. Salmonella spp. are rod-shaped, gram-negative, facultatively anaerobic bacteria belonging to the Enterobacteriaceae family.4 The genus Salmonella includes more than 2,500 serotypes within two species: S. enterica (more than 2,400 serotypes) and Salmonella bongori (20 serotypes).4,10S. enterica is a major pathogen that can infect numerous animal species, in addition to humans.10
Some Salmonella serotypes have particular host predilections. S. Dublin, Salmonella Choleraesuis, and Salmonella Gallinarum preferentially infect cattle, pigs, and chickens, respectively.4,9,10Salmonella Typhi and Salmonella Paratyphi infect only humans, causing typhoid fever. In contrast, Salmonella Typhimurium and Salmonella Enteritidis can infect a wide range of host species.
Bovine salmonellosis is caused predominantly by S. Dublin and S. Typhimurium;1,4 however, other serotypes are also capable of causing infection in cattle.1,4 In adult cattle, S. Dublin infection is common, and can be asymptomatic or characterized by abortion in pregnant cows. S. Dublin infection is also associated with fever, reduced milk production, and mild-to-moderate diarrhea. The affected animals shed S. Dublin intermittently, leading to sporadic or repeated outbreaks of disease in the herd.10 In calves, S. Dublin is associated with systemic infections, which can result in meningoencephalitis, polyarthritis, hepatitis, cholecystitis, pneumonia, splenitis, and lymphadenitis occasionally in the absence of diarrhea.4,10,11 Recently, pyelonephritis, urocystitis, ureteritis, and gangrene of the distal extremities have also been reported in calves.3,7,12
Conversely, S. Typhimurium causes enteritis and marked acute exudative diarrhea in young calves less than 2 months of age.4 Fever, anorexia, prominent diarrhea, and dehydration, which are secondary to acute necrotizing enteritis, are found in bovine S. Typhimurium infections. Disease severity and lethality were inversely proportional to the age of the affected calves. The feces are often watery, with variable amounts of mucus, fragments of the intestinal mucosa, or blood clots. Abortion is uncommon in S. Typhimurium infections.10
The diagnosis of septicemic salmonellosis is based on clinical and pathological findings and confirmed through microbiological culture and identification of S. Dublin by the polymerase chain reaction technique.6S. Dublin was identified by culture, 16S rDNA sequencing and serotyping. On immunohistochemical examination, Salmonella O9 antigen was detected in the lesions. The pathological and bacterial findings in this case were consistent with septicemic salmonellosis.11
Oral transmission can occur via the feces, contaminated food, water, or milk/colostrum. The emergence of multidrug-resistant strains is beginning to limit treatment options.1,5,15
Contributing Institution:
National Institute of Animal Health,
National Agriculture and Food Research Organization (NARO)
3-1-5Kannondai, Tsukuba, Ibaraki 305-0856, Japan
(WSC ID95)
http://www.naro.affrc.go.jp/english/niah/index.html
JPC Diagnosis:
- Liver: Hepatitis, necrotizing, multifocal, random, marked (paratyphoid nodules), with vasculitis and thrombosis.
- Bile duct, adventitia: Edema, diffuse, moderate.
JPC Comment:
It is hypothesized that there were three major steps in the evolution of Salmonella enterica subsp. enterica from its common ancestor with Escherichia coli.1 The first step involved acquisition of Salmonella pathogenicity island (SPI-1), which is present in all Salmonella species and encodes virulence factors important in establishing an intestinal infection. 1 The second step involved acquisition of Salmonella pathogenicity island 2 (SPI-2), which is present in S. enterica but lacking in S. bongori. 1 The last jump involved expansion of the host range from ectothermic vertebrates to bird and mammals, and the bacteria may have developed the ability to survive within macrophages as a mechanism to overcome the robust gastrointestinal defense mechanisms in this new host range.1
As the contributor describes, different serotypes of Salmonella enterica subsp. enterica have different host ranges and spectra of disease. Unrestricted serotypes, including Typhimurium and Enteritidis, tend to cause enteritis in young animals in a wide range of species. Host-adapted serotypes, such as Dublin and Cholerasuis, can cause either enteritis or systemic disease in their specific species.14Host-restricted serotypes, including Tyhpi, Gallniarium, and Abortisuis, tend to cause systemic infection with minimal to no enteritis in their specific species.14
|
Table 3-1. Examples of host adapted and restricted Salmonella enterica subsp. enterica serotypes. Adapted from Uzzau et al, 2000. |
|||
|
Serotype |
Natural host |
Other hosts (rare) |
|
|
Host-adapted |
Cholerasuis |
Swine |
Human |
|
Host-adapted |
Dublin |
Bovine |
Human, ovine |
|
Host-restricted |
Typhi |
Human |
- |
|
Host-restricted |
Paratyphi A, C |
Human |
- |
|
Host-restricted |
Sendai |
Human |
- |
|
Host-restricted |
Abortusovis |
Ovine |
- |
|
Host-restricted |
Gallinarum |
Poultry |
- |
|
Host-restricted |
Typhisuis |
Swine |
- |
|
Host-restricted |
Abortisequi |
Equine |
- |
Typically, bacteria which are highly adapted to a host have evolved to cause a minimal to tolerable level of disease, allowing for host survival and spread of bacterial infection.1S. enterica is unique in that high adaptation corresponds to higher virulence within the host, which mathematic models suggest has favored the development of the carrier state.1 The success of this strategy is evidenced by the proverbial disease carrier, typhoid Mary, who harbored S. Typhi in a gallstone and spread the infection to numerous individuals while working as a cook in the early 1900s.8
Salmonella possesses flagella which enable motility and uses fimbriae to adhere to the mucosal epithelial cells.13 A type III secretion system allows injection of effector proteins into the cytoplasm. 13 The bacteria is engulfed into a vacuole via receptor-mediated endocytosis or by entering through the intercellular junction complex. 13 LPS on the cell wall resists host defense mechanisms (such as opsonization) and stimulates prostaglandin synthesis. In cases of enteritis, diarrhea occurs as a result of enterocyte loss, prostaglandin E2-induced hypersecretion, and leakage from damaged mucosa. 13
In systemic salmonellosis, the bacteria invades and survives within macrophages, producing bacteremia and septicemia.13S. Dublin is unique in that it can also survive extracellularly and spread free within lymphatic fluid.13 Once in systemic circulation, bacteria are removed by fixed macrophages throughout the body, including in the liver, spleen, kidney, and bone marrow, producing paratyphoid granulomas as seen in this case.13 In severe cases, septicemia may be fatal. 13
As the contributor describes, S. Dublin causes severe acute disease in calves characterized by endotoxemia with depression, respiratory distress, and death. In a recent review of 57 infected Holstein calves less than 6 months of age, the most consistent lesions were neutrophilic infiltrates in the alveolar capillaries and septae and necrosuppurative and histiocytic hepatitis with paratyphoid granulomas.11 In approximately half of the cases, neutrophilic infiltrates were observed in the lymph nodes and marginal zone of the spleen.11 Key features which differentiated S. Dublin from systemic E. coli infection were paratyphoid nodules in the liver and age, with E. coli endotoxemia primarily affecting calves less than two weeks of age and S. Dublin affecting older calves.11
The moderator provided a general rule of thumb for two gross necropsy findings highly suggestive of S. Dublin infection in calves: icterus and gall bladder edema with mucosal pseudomembrane formation.
References:
- Baumler AJ, Tsolis RM, Ficht TA, Adams LG. Evolution of Host Adaptation in Salmonella enterica. Infection and Immunity. 1998: 4579-4587.
- Casaux ML, Caffarena RD, Schild CO, Giannitti F, Riet-Correa F, Fraga M: Antibiotic resistance in Salmonella enterica isolated from dairy calves in Uruguay. Braz J Microbiol. 2019; 50(4):1139-1144.
- Costa RA, Casaux ML, Caffarena RD, Macías-Rioseco M, Schild CO, Fraga M, Riet-Correa F, Giannitti F: Urocystitis and ureteritis in Holstein calves with septicaemia caused by Salmonella enterica serotype Dublin. J Comp Pathol. 2018; 164:32-36.
- Costa LF, Paixão TA, Tsolis RM, Bäumler AJ, Santos RL: Salmonellosis in cattle: advantages of being an experimental model. Res Vet Sci. 2012; 93(1):1-6.
- Estevez MB, Casaux ML, Fraga M, Faccio R, Alborés S: Biogenic silver nanoparticles as a strategy in the fight against multi-resistant Salmonella enterica isolated from dairy calves. Front Bioeng Biotechnol. 2021; 9:644014.
- Guizelini CC, Tutija JF, Morais DR, Bacha FB, Ramos CAN, Leal CRB, Zaquetti ME, Lemos RAA: Outbreak investigation of septicemic salmonellosis in calves. J Infect Dev Ctries. 2020; 14(1):104-108.
- Loeb E, Toussaint MJ, Rutten VP, Koeman JP: Dry gangrene of the extremities in calves associated with Salmonella dublin infection; a possible immune-mediated reaction. J Comp Pathol. 2006; 134(4):366-369.
- Marineli F, Tsoucalas G, Karamanou M, Androustous G. Mary Mllon (1869-1938) and the history of typhoid fever. Ann Gastroenterol. 2013; 26(2): 132-134.
- Meneguzzi M, Pissetti C, Rebelatto R, Trachsel J, Kuchiishi SS, Reis AT, Guedes RMC, Leão JA, Reichen C, Kich JD: Re-emergence of salmonellosis in hog farms: outbreak and bacteriological characterization. Microorganisms. 2021; 9(5):947.
- Nielsen LR: Review of pathogenesis and diagnostic methods of immediate relevance for epidemiology and control of Salmonella Dublin in cattle. Vet Microbiol. 2013; 162(1):1-9.
- Pecoraro HL, Thompson B, Duhamel GE: Histopathology case definition of naturally acquired Salmonella enterica serovar Dublin infection in young Holstein cattle in the northeastern United States. J Vet Diagn Invest. 2017; 29(6):860-864.
- Taghipur Bazargani T, Khodakaram-Tafti A, Ashrafi I, Abbassi AM: Giant hydronephrosis and secondary pyelonephritis induced by Salmonella Dublin in a Holstein calf. Iran J Vet Res. 2015; 16(1):114-116.
Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016: 167-176. - Uzzau S, Brown DJ, Wallis T, et al. Host adapted serotypes of Salmonella enterica. Epidemiol Infect. 2000 (125): 229-255.
- Wang X, Biswas S, Paudyal N, Pan H, Li X, Fang W, Yue M: Antibiotic resistance in Salmonella Typhimurium isolates recovered from the food chain through national antimicrobial resistance monitoring system between 1996 and 2016. Front Microbiol. 2019; 10:985.