Signalment:
16-year-old, neutered male, mixed breed horse (
Equus caballus).The horse was donated and become part of a research study. The horse received a
commercially available
Escherichia coli O55:B5 lipopolysaccharide (LPS) solution infused via
intravenous catheter at a dose of 5 ng/kg/hr for 8 hours. Twenty-four hours later the horse was
given 5g/kg of oligofructose (OF) via a nasogastric tube and was euthanized 27 hours later with
an Obel laminitis score of 2+.
Gross Description:
Grossly all tissues were within normal limits. Laminar tissue was obtained
by sectioning with a bandsaw as previously described.1 Mid-dorsal laminar sections were
trimmed into 2 cm +�-� 1 cm +�-� 0.5 cm strips using a scalpel, formalin-fixed and then processed
routinely.
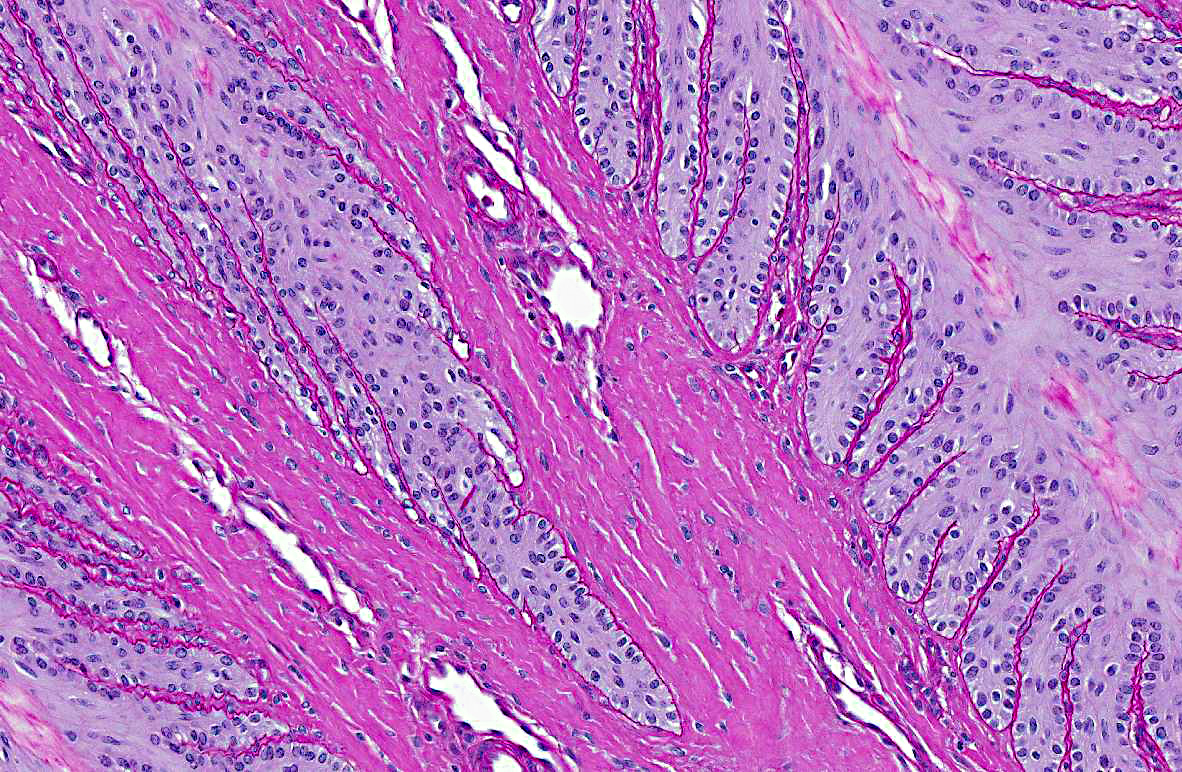
Histopathologic Description:
Slides were stained with periodic acid-Schiff preparation to
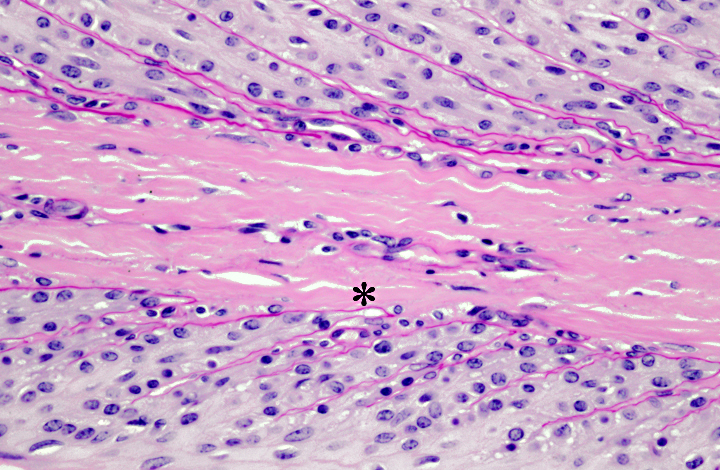
highlight the basement membranes. Histologically, there is tapering and retraction of the
secondary epidermal lamina leaving empty sleeves of basement membrane trailing off the tips of
the secondary epidermal lamina. There is also some retraction of the basement membrane and
secondary dermal lamina from between the secondary epidermal lamina, which creates the
appearance of a thicker primary epidermal lamina.
Morphologic Diagnosis:
Multifocal degeneration and loss of epidermal lamina.
Condition:
Laminitis
Contributor Comment:
In horses, the hoof is attached to the underlying distal (third) phalanx
(P3) by the interdigitation of epidermal and dermal lamina. Laminitis refers to separation of the
epidermal and dermal lamina in the hoof, which results in lameness and in chronic cases, rotation
of P3. Laminitis can be caused by a variety of insults, including, but not limited to: obesity,
endocrinopathies, colic, sepsis, toxemia, diarrhea, shock, lush pastures, excess carbohydrates,
drug therapy, intense training, and black walnut shavings.(3,4) Laminitis is typically induced
experimentally with oligofructose. This horse was involved in a research project investigating
the -�-�two-hit hypothesis, which proposes that sequential exposure to inflammatory stimuli (LPS
and OF) can exacerbate the laminitic response. Clinically the degree of laminitis (Obel score)
has been correlated to the degree of histologic lesions using Politts grading scheme.(3)
The pathogenesis of laminitis remains complex and poorly understood, but matrix
metalloproteinases are present at increased levels in laminitic tissues and are thought to mediate
the dissolution of cell-cell and cell-basement membrane adhesion.
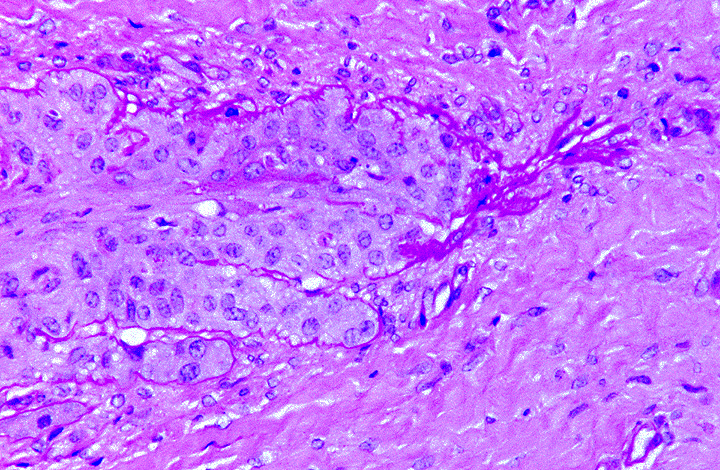
The earliest changes involve transformation of the normally club-shaped ends of the secondary
epidermal lamina with elongation and attenuation of the tips of the secondary epidermal lamina
and detachment from the underlying basement membrane (Grade I). Where the epidermal cells
detach from the basement membrane, small teat-shaped bubbles may form. In Grade II lesions,
these changes progress and there is retraction of the basement membrane and secondary dermal
lamina from between the bases of the secondary epidermal lamina.(4) As the dermal lamina are
retracted, the epidermal cells become further from their blood supply, predisposing to subsequent
ischemia.(2) Retraction of the capillaries also results in increased resistance to blood flow, which
is noticed clinically as -�-�bounding pulses. These changes in blood flow can also result in
arteriovenous anastomoses or shunts.(3,4) In grade III lesions there is almost complete separation
of the epidermal lamina from the basement membrane with loss of distinction between primary
and secondary epidermal lamina. Ultimately there is retraction of the tip of the primary
epidermal lamina as well.(4) The lesions in this case are most consistent with grade II laminitis.
JPC Diagnosis:
Hoof lamina: Epidermal laminar degeneration and necrosis with multifocal
basement membrane retraction and edema.
Conference Comment:
As the contributor states, the pathogenesis of equine laminitis is
complex and not fully understood. Historically, laminitis has been thought to be due to an
ischemic event caused by a vascular condition that constricted blood flow to the hoof.(2) Recent
research suggests that degeneration of the primary epidermal lamellae and basement membrane
loss may be the initial lesion, with vascular events and subsequent ischemia being an important
consequence of the initial laminar degeneration. This enzymatic theory of laminitis is based
on the findings of significantly increased amounts of matrix metalloproteinase-2 (MMP-2 aka
gelatinase A) and matrix metalloproteinase-9 (MMP-9 aka gelatinase B) in lamellar tissues
affected by laminitis, as well as the finding that in the developmental phase of laminitis, vessels
in the feet are actually dilated rather than constricted. MMPs are found in normal lamellar
tissues and are thought to play a role in the required remodeling of the epidermal lamellae that
occurs as a normal part of hoof growth. MMPs are produced locally, and function to release
epidermal cell-to-cell and cell-to-basement membrane adhesions, maintaining the correct shape
and orientation of the hoof lamellae as the hoof grows. The increase of MMP-2 and MMP-9 and
the subsequent destruction of the lamellar attachment apparatus has been shown to be a key
feature in acute laminitis, although the trigger factors have yet to be elucidated. Interestingly,
epidermal cells of some non-equine species, including humans, readily increase their production
of MMPs when exposed to cytokines such as TNF, IL-1, and TGH-1; however, in-vitro studies
have shown that equine MMPs are not activated on exposure to these cytokines. Additionally,
laminitic changes in equine lamellae have not been triggered experimentally by the
administration of endotoxin, prostaglandins, black walnut extract, or anaerobic conditions. The
one exception is a factor present in the supernatant from cultures of
Streptococcus bovis isolated
from the horse cecum; this factor has been found to activate equine hoof MMP-2 and has
experimentally resulted in lamellar separation. Thus the
S. bovis MMP activator may play a role
in naturally-occurring carbohydrate-overload laminitis.(2) Perhaps with continued research, the
complete pathogenesis of equine laminitis will finally be revealed.
References:
1. Pollitt CC. Basement membrane pathology: a feature of acute equine laminitis.Â
Equine Vet J. 1996;28:38-46.
2. Pollitt CC. Laminitis pathophysiology. In: Floyd AE, Mansmann RA, eds.Â
Equine Podiatry. St. Louis, MO: Saunders Elsevier; 2007.
3. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie MG, ed.Â
Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Toronto, CA: Saunders Elsevier; 2007:553-781.
4. Politt CC. Equine laminitis.Â
Clinical Techniques in Equine Practice. 2004;3:34-44.