CASE 1: B-5125-19 (4156736-00)
Signalment:
3-year-old, gender unknown, domestic bovine, Bos taurus
History:
The animal was dyspneic and sent to slaughter. The head and lungs, which appeared abnormal, were collected at the abattoir and submitted to our diagnostic lab for further examination.
Gross Pathology:
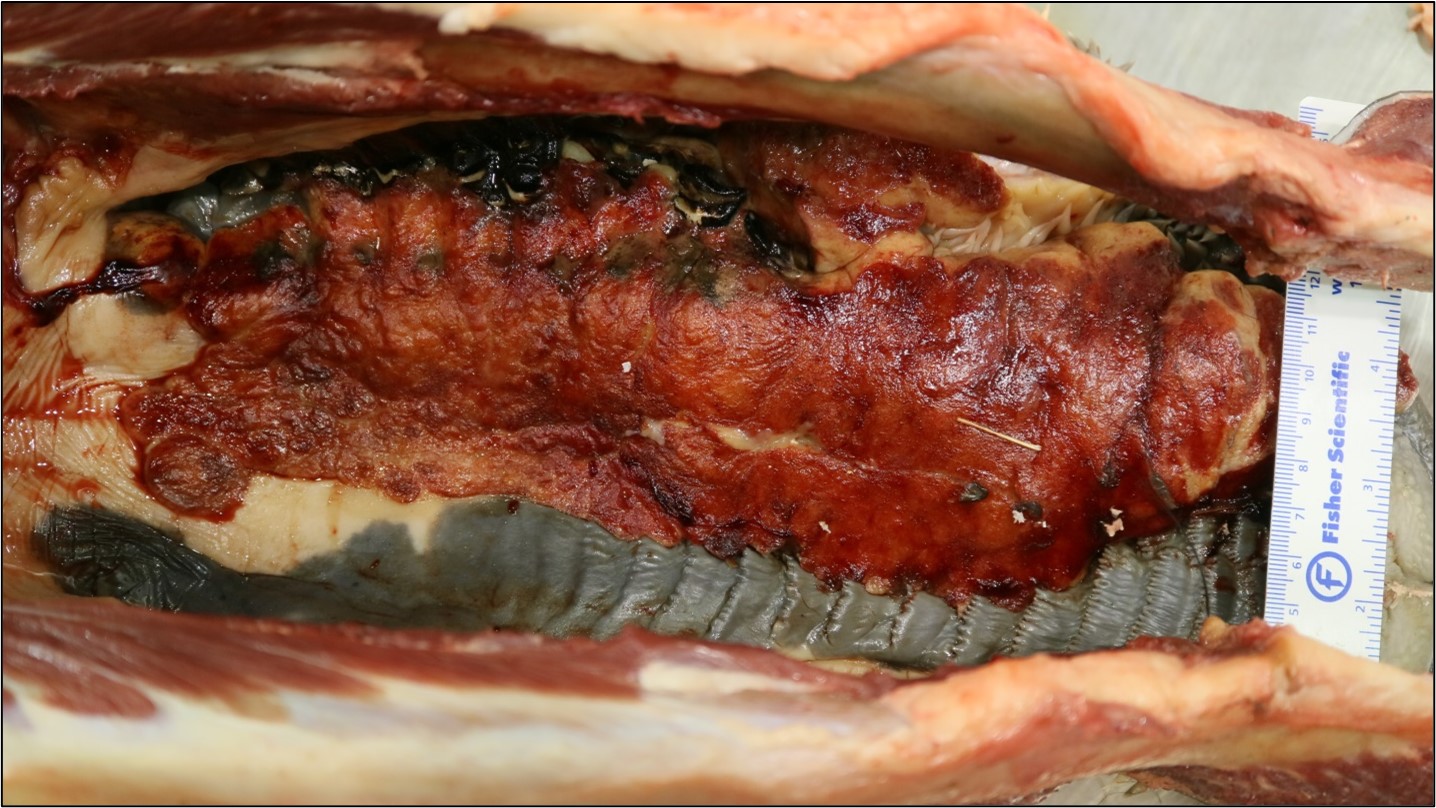
The oral mucosa and soft tissues covering most of the hard and soft palate, extending from the rostral maxilla into the pharynx, are extensively ulcerated and markedly thickened by a 2-3 cm thick layer of yellow-tan, solid, slightly firm, homogenous tissue. This tissue often abuts the underlying maxillary bone but does not invade it. Small amounts of cloudy, tan exudate could be expressed from this tissue. Numerous, often coalescing, nodular proliferations of similar tissue, ranging in size from 2-6 cm in greatest diameter are also noted in the buccal mucosa (sections submitted for your examination) and have effaced retropharyngeal and submandibular lymph nodes. Many (> 100) similar, 2-4 cm in greatest diameter, nodules are scattered throughout both the right and left lung lobes.
Laboratory results:
Aerobic culture of samples of affected oral mucosa isolated a heavy growth of Actinobacillus lignieresii.
Microscopic description:
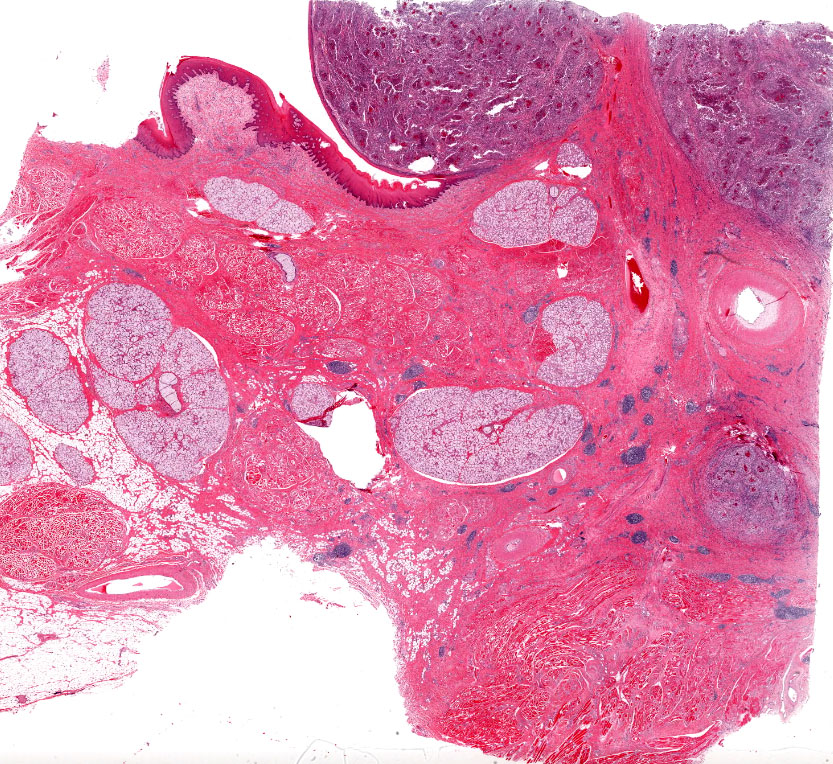
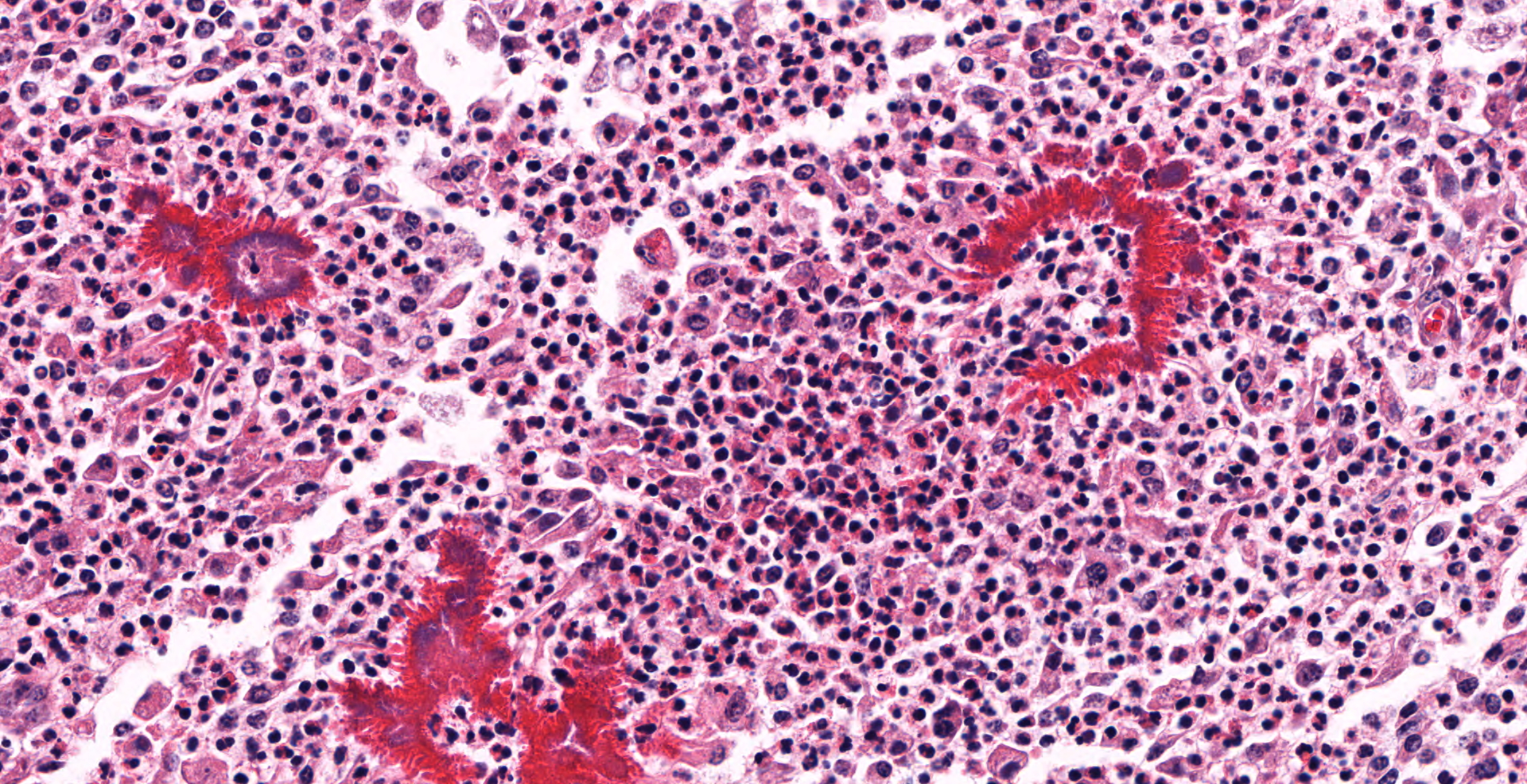
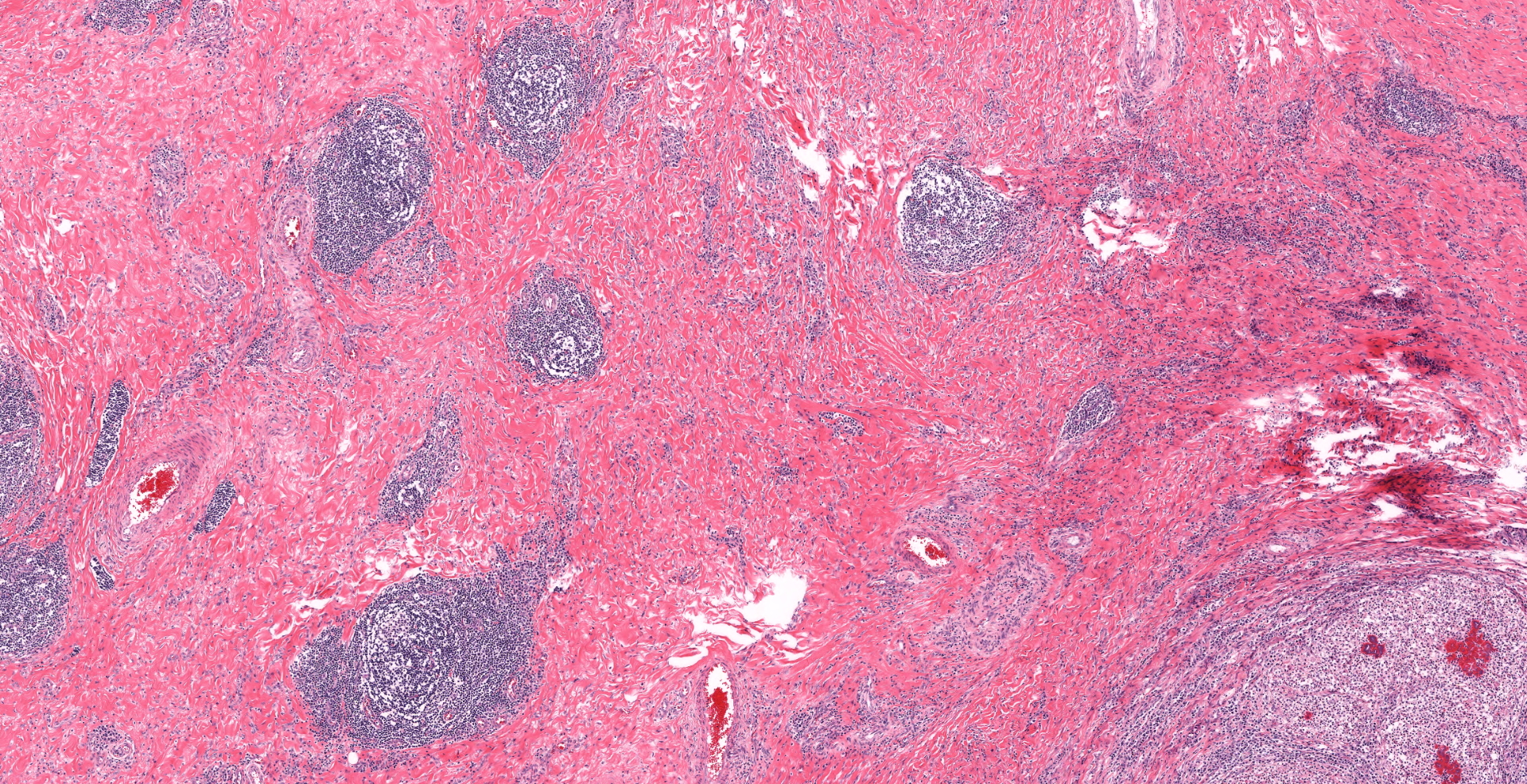
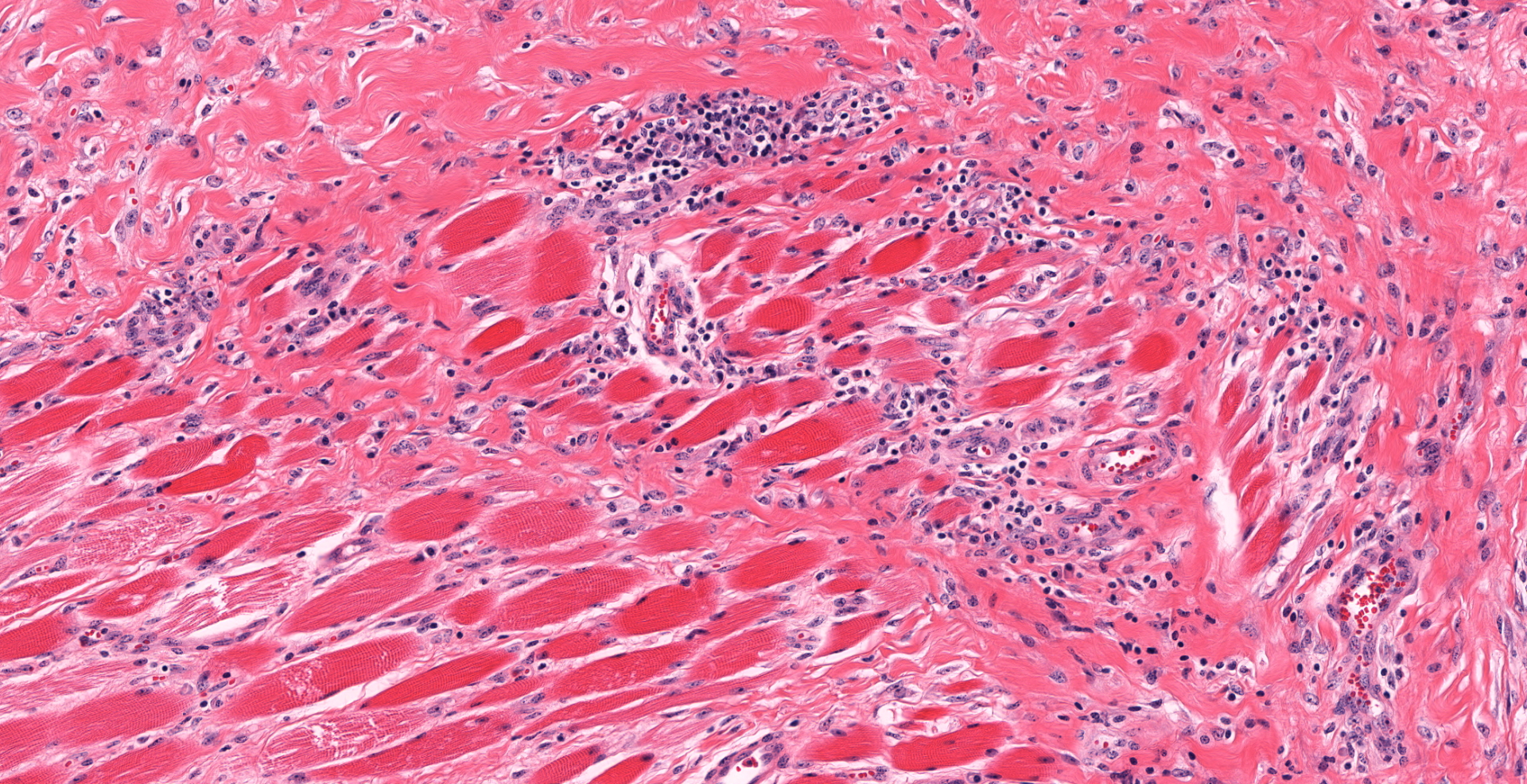
The buccal submucosa is raised and contains densely cellular, nodular aggregates of neutrophils and numerous epithelioid macrophages intermingled with fewer plasma cells and lymphocytes. Scattered within inflammatory infiltrates are numerous small clusters of fine, gram negative, coccobacilli which are surrounded by a dense, sometimes undulating layer of hypereosinophilic material composed of tiny, outwardly radiating, blunt, club-like projections (consistent with Splendore-Hoeppli material). The surrounding and deeper submucosa is moderately expanded with large amounts of dense, mature fibrous tissue containing frequent scattered small dense aggregates of lymphocytes and plasma cells. Sections of the regional lymph nodes are largely effaced by similar nodular pyogranulomatous aggregates. The nodules noted in the lung are similar in appearance.
Contributor's morphologic diagnosis:
Oral mucosa: Severe, nodular, pyogranulomatous, stomatitis with intralesional gram negative, coccobacilli surrounded by Splendore-Hoeppli material ("club colonies" or "sulfur granules")
Contributor's comment:
The gross and microscopic appearance of this oral lesion is compatible with a severe presentation of oral actinobacillosis (often referred to as "wooden tongue") due to infection with Actinobacillus lignieresii. This gram-negative bacterium is part of the normal oral and rumen flora in cattle and infections have been reported in both beef and dairy herds. Trauma to the glossal and/or oral mucosa, often attributed to the ingestion of rough plants/forages or teeth abrasions, is thought the primary route of bacterial infection in most affected animals. The most common or "typical" presentation of actinobacillosis in cattle is due to bacterial colonization of glossal, oral and/or peri-oral soft tissues resulting in dense foci of pyogranulomatous inflammation or abscesses in which are embedded numerous small dense aggregates of gram-negative bacteria. Bacterial colonies are surrounded by a dense, rim of hypereosinophilic, material consistent with what has been termed Splendore-Hoeppli material. The latter consists of a combination of immune complexes, tissue debris and fibrin and these bacterial aggregates have been referred to as "club colonies" or "sulfur" granules. As in this case, the surrounding soft tissues are often expanded and replaced by marked fibrosis and granulation tissue, which, when involving the tongue, gives it a very firm, immobile, "wooden" appearance .2,8,10
Spread of Actinobacillus lignieresii infection within soft tissues via lymphatics commonly results in "atypical" forms of the disease characterized by pyogranulomatous inflammation involving regional parotid, retropharyngeal, submandibular, and occasionally, more distant lymph nodes. Infection may extend into the overlying skin which becomes ulcerated and forms draining lesions. Cutaneous, esophageal, lung, and peritoneal pyogranulomas and lesions within the walls of the forestomach, have also rarely been reported in atypical forms of A. lignieresii infection, with and without concurrent, oral lesions.8,9 Hematogenous spread is also likely involved in some cases of disseminated infection. Stress associated with transportation of young cattle was suspected in be a contributing factor in the development of atypical infection in one report.4 Outbreaks of the disease in cattle have been reported in South America that could not be related to the type of forage fed. Previous viral disease (ie. foot-and-mouth disease) resulting in oral lesions, stress and possibly some degree of immunocompromise was suspected to be a factor in the increased incidence of actinobacillosis noted.2
In this case, oral lesions were very severe, involving much of the oral mucosa. Infection was also well established in numerous retropharyngeal, parotid and submandibular lymph nodes and myriad embolic pyogranulomas were present throughout the lung. Grossly, these lesions resembled a neoplastic process (such as lymphoma). An underlying cause of such extensive and widespread infection in this case was not identified, but only the head and lungs had been submitted for examination. The dyspnea noted was largely attributed to stenosis of the pharynx due to marked retropharyngeal and pharyngeal lymph node enlargement.
The main differential diagnosis for this oral lesion would be Actinomyces bovis infection. The latter is a gram-positive bacteria that also induces oral pyogranulomatous inflammation. This organism typically also invades the underlying bone and causes areas of chronic osteomyelitis ("lumpy jaw" or actinomycosis), findings which were not present in this case. Nocardia sp. infection may also produce similar lesions.2
Caffarena, et al2 indicated that Actinobacillus sp. infection of lymph nodes of the head of cattle in South America is a relatively common finding in abattoirs that grossly may be mistaken for tuberculosis. Microscopic evaluation, cytologic evaluation of exudates, and/or ancillary bacterial testing would be required to differentiate these conditions.
Although rare, A. lignieresii infection is potentially zoonotic. In humans, both acute fatal systemic and chronic localized infections have been reported.6,9
Contributing Institution:
Atlantic Veterinary College
University of Prince Edward Island
JPC diagnosis:
Oral mucosa: Stomatitis, pyogranulomatous, multifocal to coalescing, chronic, severe, with fibrosis, and colonies of coccobacilli surrounded by Splendore-Hoeppli material.
JPC comment:
The moderator reviewed a variety of pathogens most often associated with Splendore-Hoeppli material, which included Actinobacillus lingnieresii, Actinomyces bovis, Nocardia spp, and Staphylococcus aureus associated with botryomycosis. The use of gram stains and acid fast stains are most helpful for differentiating between these bacteria.
The contributor provides a succinct summary of this disease of ruminants, and rarely dogs, horses, and rats. Actinobacillus lignieresii is a member of the family Pasteurellaceae, and is closely related to A. pleuropneumoniae, A. suis, and A. equuli. While Actinobacillus spp are often associated with RTX-type toxins, A. lignieresii contains the genes that code for Apx, but lacks a functioning promoter, and so does not elaborate this class of toxin. However, they also produce urease, which releases ammonia from urea. Higher levels of ammonia are chemotactic and activating for neutrophils and macrophages, inhibit phagolysosome fusion, and the increased pH within phagolysosomes reduces the effectiveness of various acid hydrolases. This bacterium also has a capsule that is antiphagocytic and interferes with the membrane attack complex of the complement system, in addition to the lipopolysaccharide (LPS) cell wall.3
As noted by the contributor, the Splendore-Hoeppli phenomenon is thought to be an aggregation of protein deposits from antigen-antibody reaction and debris from inflammatory cells. It tends to be arranged in layers of varying thickness but uniform appearance, conferring the radiating attribute to the projecting material.5 While Splendore-Hoeppli material is most often visualized in histologic sections, it can also be observed in cytologic specimens. In a case of human facial actinomycosis, aspirate smears showed round to ovoid 100-200 mm three-dimensional structures with ill-defined, fuzzy borders. These structures were mats of filamentous Actinomyces spp bacteria intermixed with elongated rectangular, rhomboid to rounded, dense, glassy dark green-blue (Diff-Quik) or intensely orange-pink (Papanicoloau stain) crystals, measuring 10-40 x 6-12 mm and found singly or in clusters.1
One of the people who first described this host response to antigen was Reinhard Hoeppli, a Swiss-German physician who was a parasitologist by training. He had trained at the Institute of Pathology at Kiel University and then joined the Institut fur Sciffs- und Tropenkraheiten (Institute for Maritime and Tropical Disease) in 1921 as an assistant to Fredrich Fulleborn, the head of the helminthology department. Hoeppli's description of the host response to schistosome eggs embedded in the tissue of a rabbit was the first written in English and was subsequently found to be quite similar to the description of the host reaction elicited by Sporothrix schenckii by Splendore in 1908. He eventually renounced his German citizenship and asserted his Swiss citizenship by right of descent. During World War II, he was named the Swiss Honorary Consul in Beijing, which was under Japanese control. He ultimately became acquainted with an Englishman, Sir Edmond Backhouse, an eccentric but charming conversationalist. Hoeppli encouraged Backhouse to record his bizarre and scandalous recollections into two manuscripts, which Hoeppli held until his death in 1973. Unfortunately for Hoeppli, his estate ultimately sent the manuscripts to an Oxford historian, Hugh Trevor-Roper, who found Backhouse to be a forger, charlatan, and fantasist with the documents holding no historical value. In the process of dismantling the story of Sir Edmond Backhouse, Trevor-Roper was acerbic and disparaging toward Hoeppli, ultimately tarnishing the reputation of a fascinating scientist.7
References:
- Aragao A, Bimer J, Barkan GA, Pambuccian SE. Splendore-Hoeppli phenomenon in a fine needle aspirate of cervicofacial actinomycosis. Diagnostic Cytopathology. 2018;47:238-243.
- Caffarena RD, Rabaza A, Casaux L, Rioseco MM, Schlid CA, Monesiglio C, Fraga M, Giannitti F, Reit-Correa F. Natural lymphatic ("atypical") actinobacillosis in cattle caused by Actinobacillus lignieresii. J Vet Diagn Invest. 2018; 30(2): 218-225.
- Fenwick BW, Woolums AR. Pasteurellaceae: Actinobacillus. In: McVey DS, Kennedy M, Chenappa MM, eds. Veterinary Microbiology, 3rd Ed. Ames, IA: John Wiley and Sons. 2013:108-114.
- Kasuya K, Manchanayake T, Uenoyama K, Kawa S, Takayama K, Imai N, Shibahara T. Multifocal suppurative granuloma caused by Actinobacillus lignieresii in the peritoneum of a beef steer. J Vet Med Sci. 2017; 79(1): 65-67.
- Martinez-Giron R, Pantanowitz L. "Splendore Hoeppli" phenomenon. Diagnostic Cytopathology. 2020;48(12)1316-1317.
- McDaniel CJ, Cardwell DM, Moeller RB, Gray GC. Humans and cattle: A review of bovine zoonoses. Vector-Borne and Zoonotic Diseases. 2014; 14(1): 1-19.
- Morley NJ. Reinhard Hoeppli (1893-1973): The life and curious afterlife of a distinguished parasitologist. Journal of Medical Biography. 2019. doi:10.1177/0967772019877608.
- OM Radostits, CC Gay, KW Hinchcliff, and PD Constable. In Veterinary Medicine, 10th ed. Saunders Elsevier Publishing. 2007 :1046-1047.
- Orda R. Actinobacillus lignieresii human infection. J Royal Soc of Med. 1980; 73: 295-297.
- Uzal F, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, 6th ed. St' Louis, Missouri: Elsevier; 2016: Vol 2: 18-19.