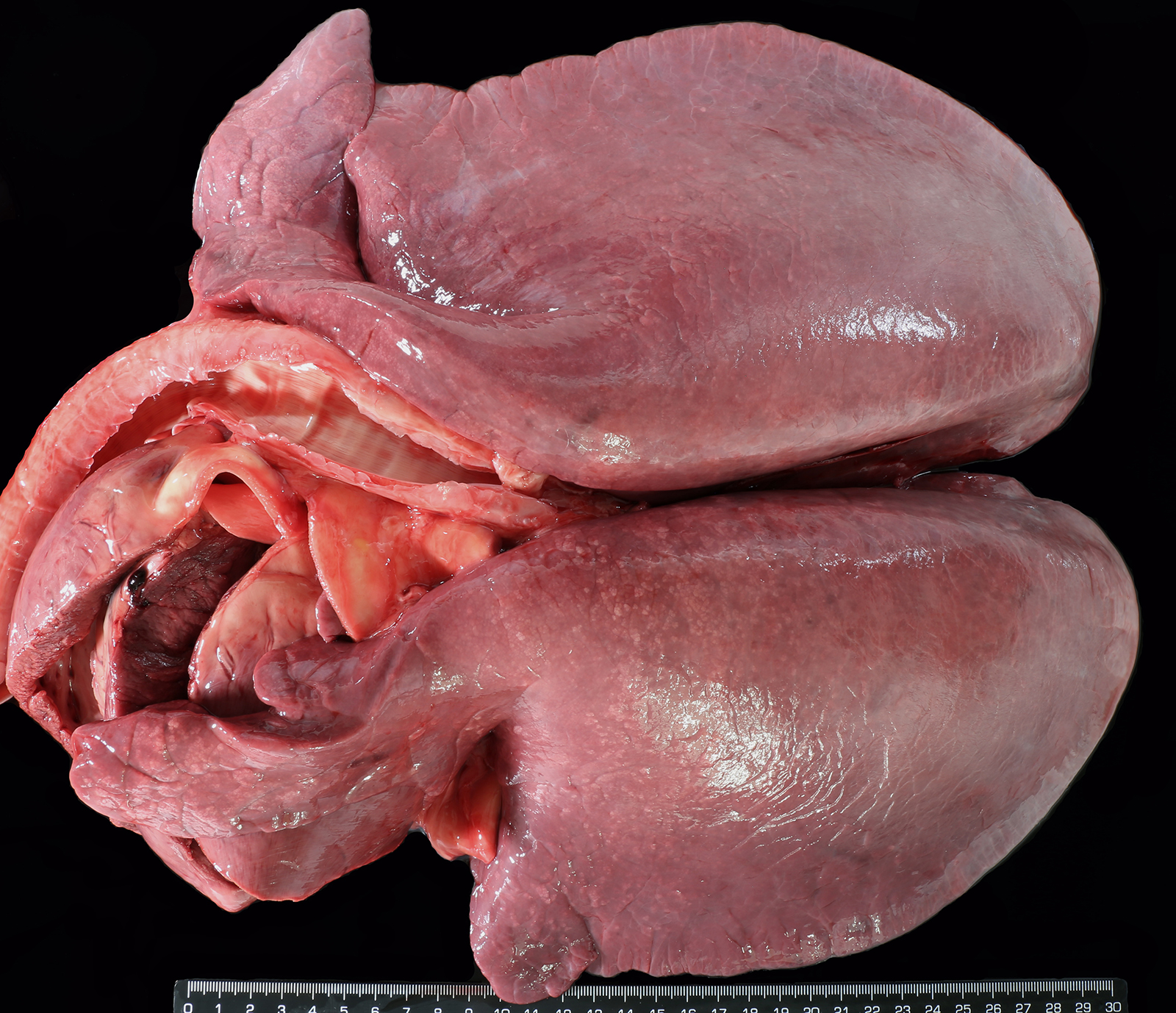
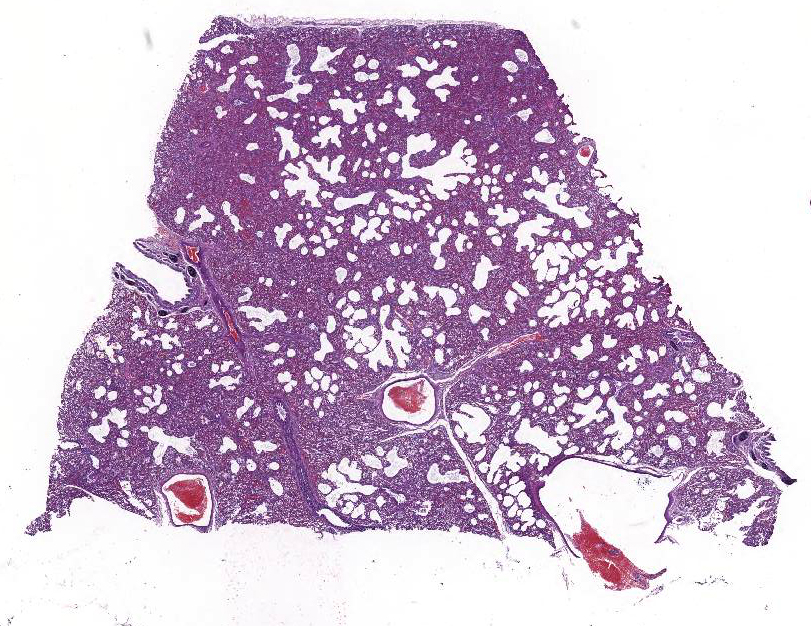
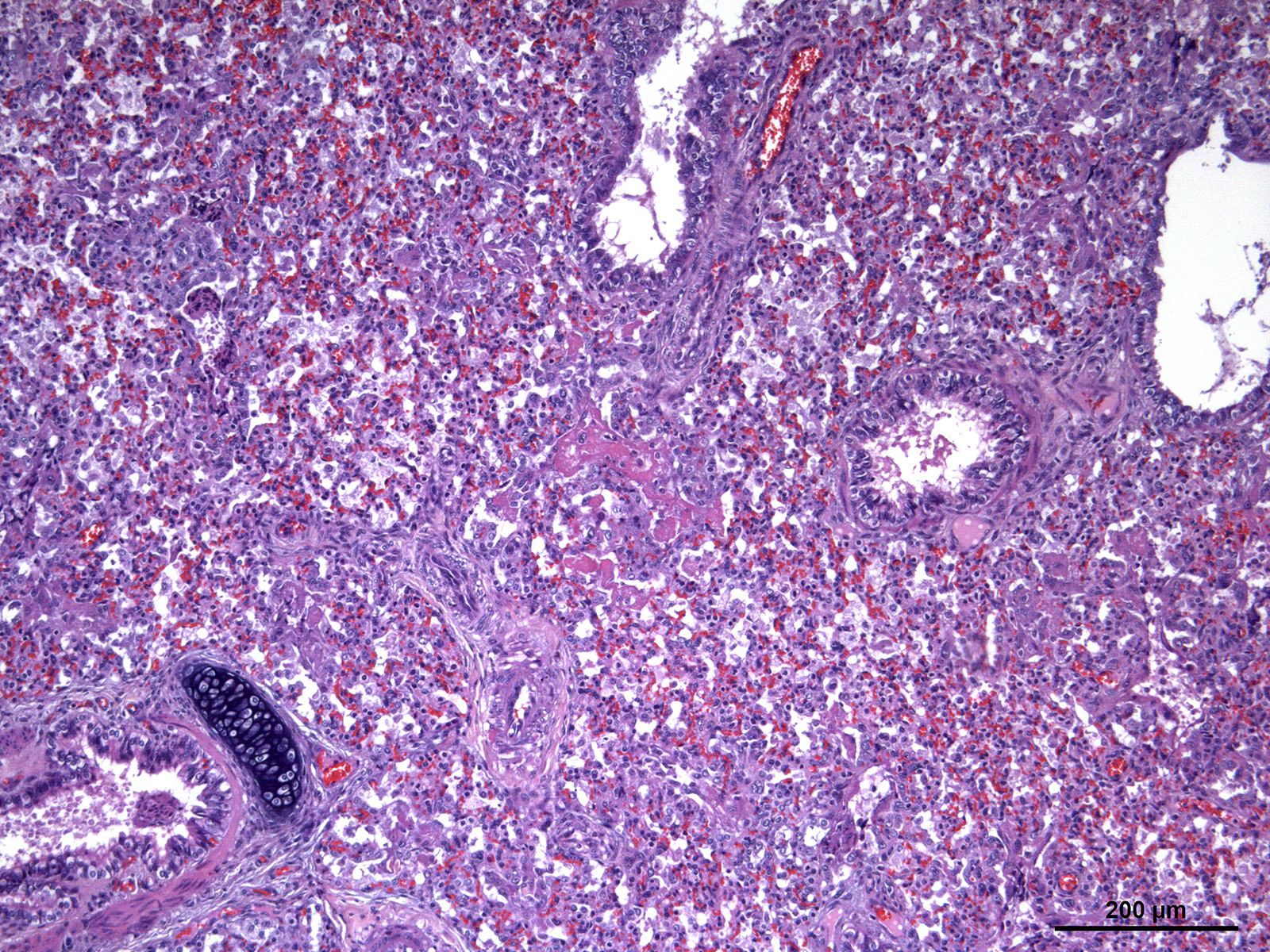
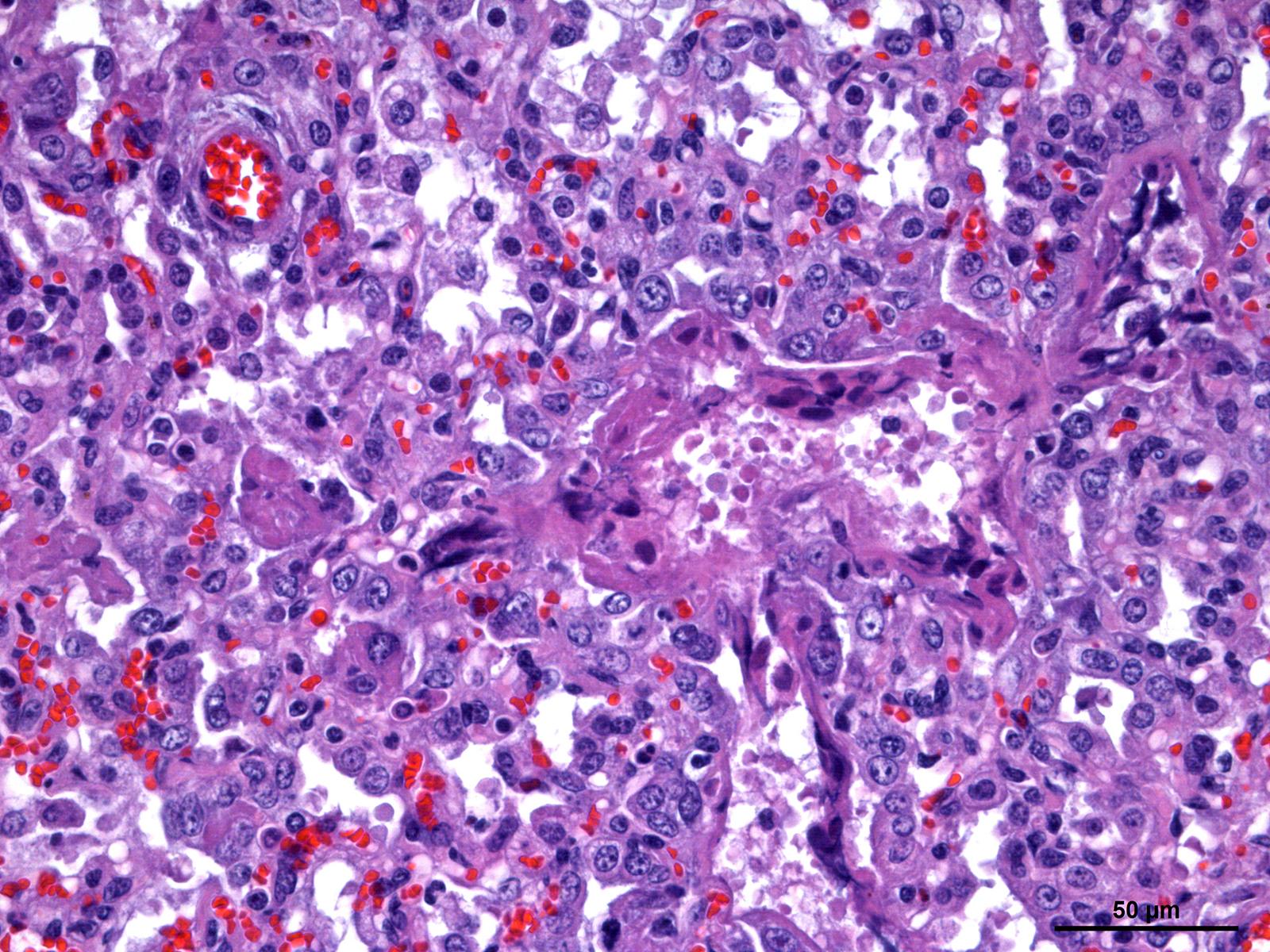
Foal was weak but ambulatory with expiratory dyspnea and tachypnea since birth and showed an elevated respiratory and cardiac rate and fever. Mucous membranes were hyperemic with multifocal petechiation of the oral mucous membranes and in the inner side of the pinna. The capillary refill time was less than 2 seconds. Pulmonary auscultation revealed snoring, wheezing, and crepitation with a diffuse and moderate alveolar pattern on thoracic radiographs. Supportive care and treatment, including positive pressure ventilation, were pursued without success and the foal was eventually euthanized.