Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 13
8 January 2020
MAJ Sarah Cudd
CASE IV: Rab 51 (JPC 4118093).
Signalment: Adult (age unknown), female entire, wild European rabbit, Oryctolagus cuniculus.
History: This rabbit is from a group of wild rabbits shot for population control and subsequently submitted for post mortem examination for research and teaching purposes (institutional Ethics and Welfare Committee reference: CR240).
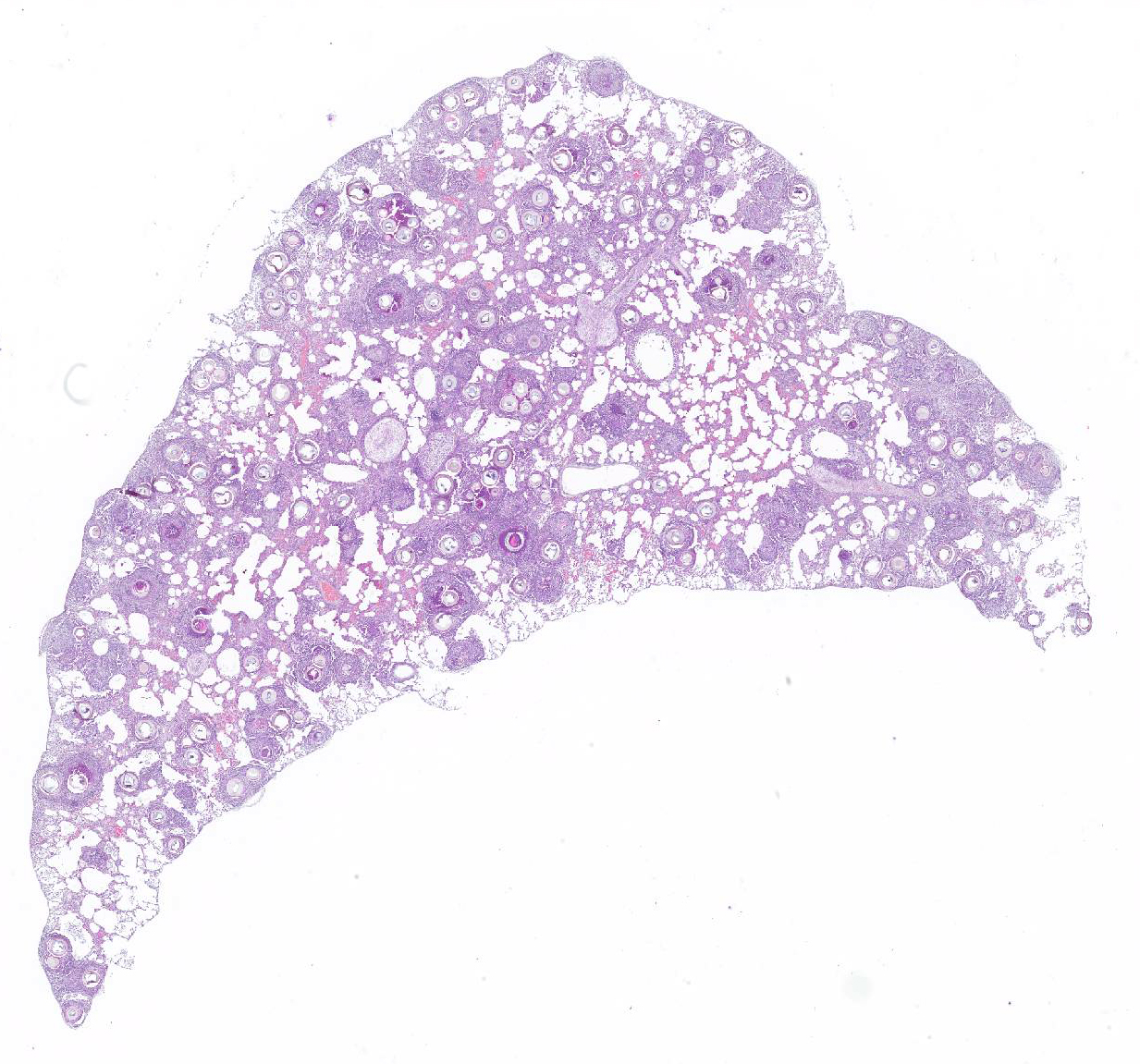
Gross Pathology: The lungs have a light brown to pink surface and a firm, gritty texture. A large number of coalescing granulomas that measure up to 1 mm in diameter expand the pulmonary parenchyma on cut surface (Fig. 1). Tracheobronchial lymph nodes have smooth, homogenous and cream capsular and cut surfaces and measure approximately 8x5x5 mm.
There are multifocal, randomly distributed, well demarcated, cream foci within the liver parenchyma and level with or slightly raised from the capsular surface. These foci measure up to 2 mm in diameter (gross and microscopic findings confirmed concurrent Eimeria stiedae infection).
Laboratory results:
None available.
Microscopic Description:
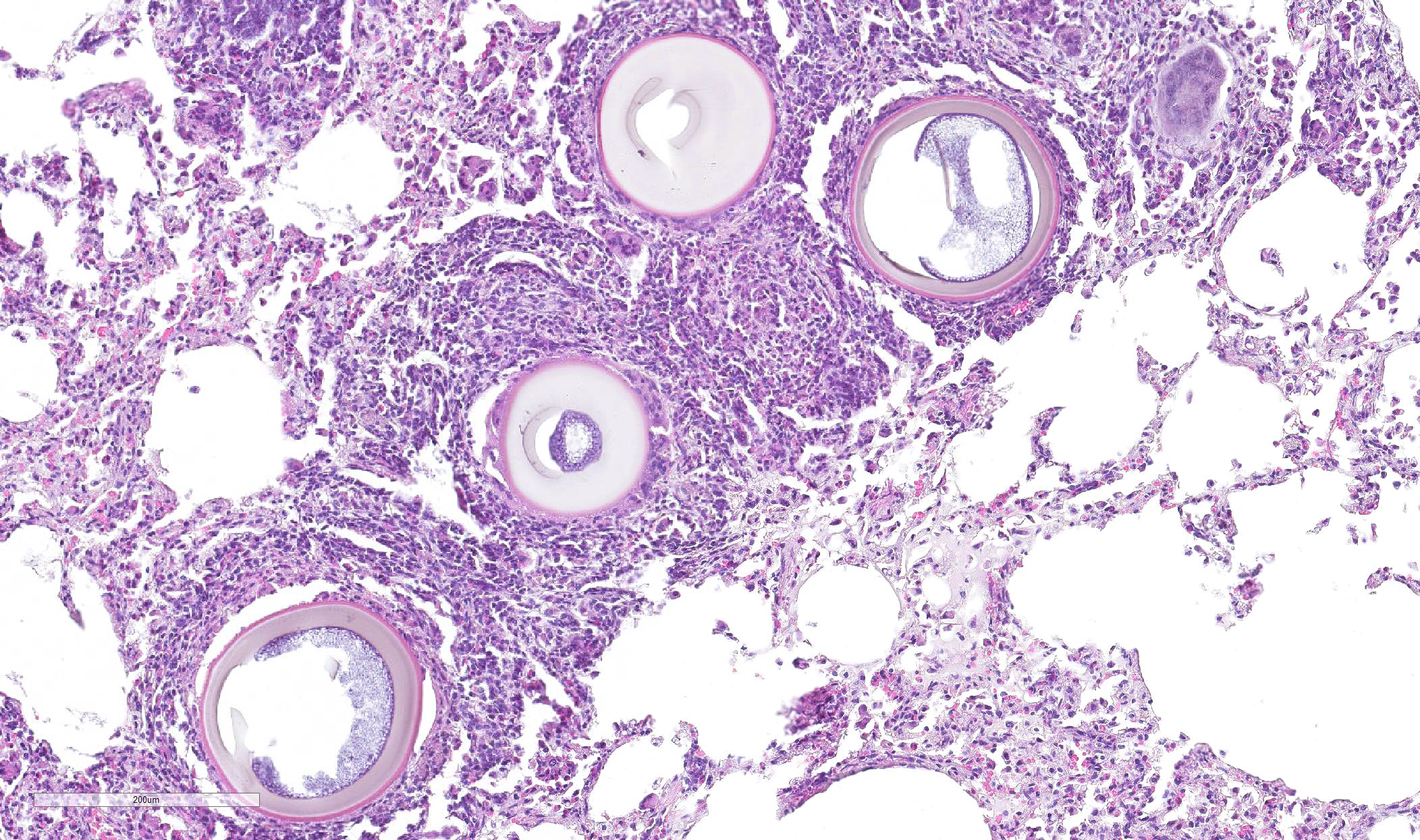
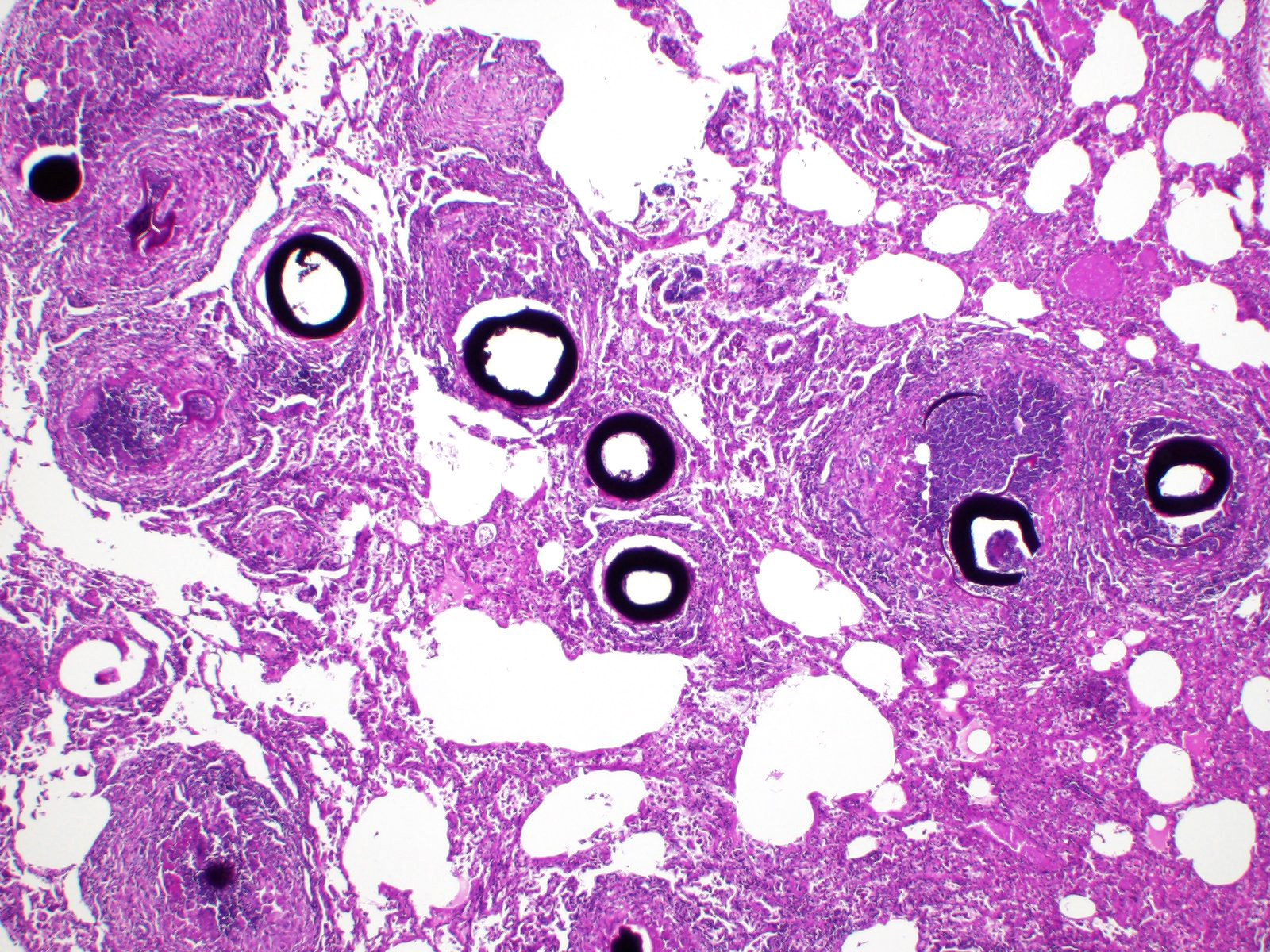
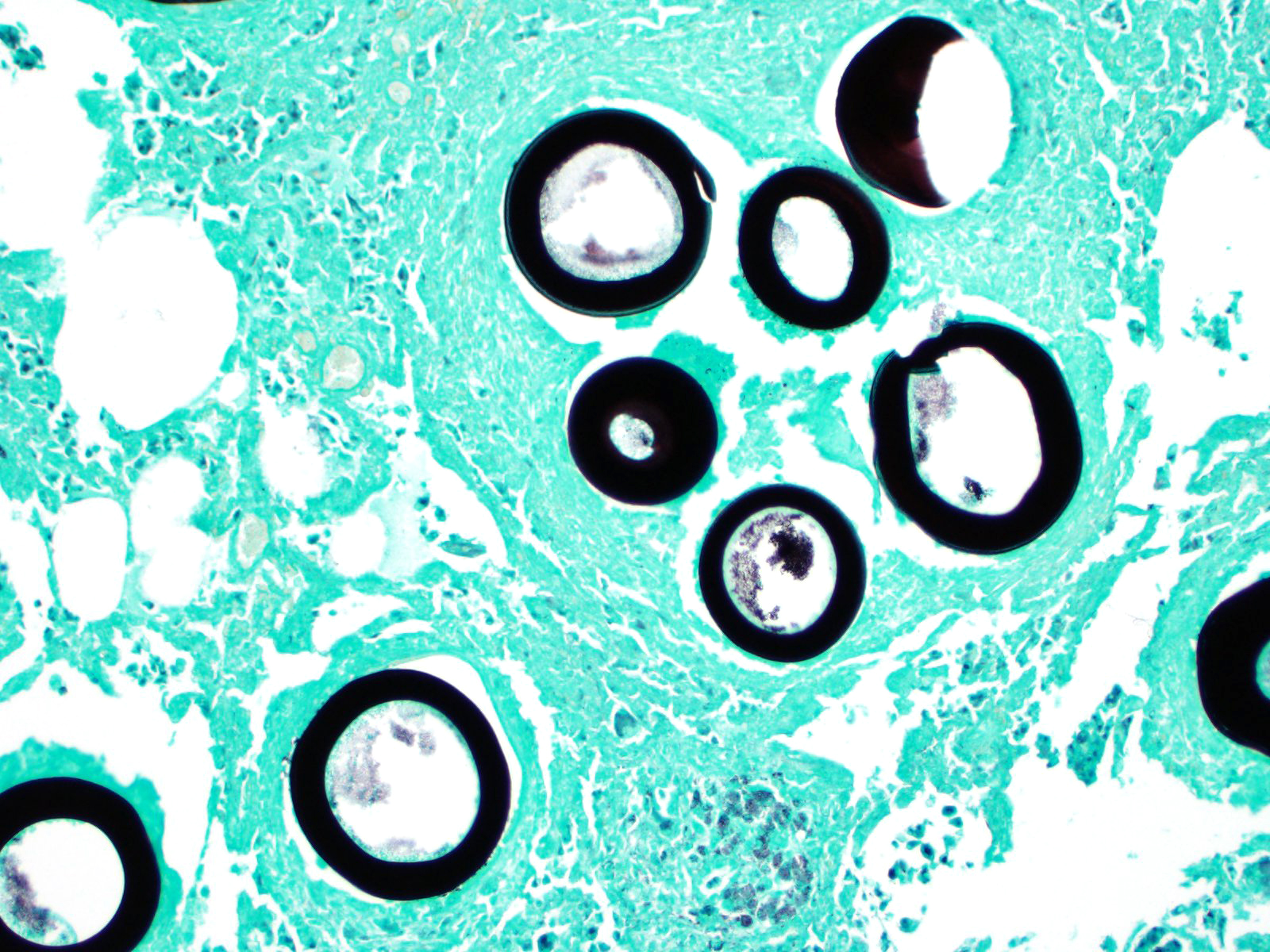
Greater than 90% of the pulmonary parenchyma is expanded by a large number of multifocal to coalescing granulomas surrounding thick walled adiaspores. Adiaspores measure 200-300mm in diameter and have a wall which consists of a bright eosinophilic, approximately 3mm thick outer layer, a 30-40mm thick pale eosinophilic middle layer and a variably prominent, 2-3mm thick, basophilic inner layer. The adiaspore wall surrounds a foamy, variably pale basophilic core. Large numbers of concentrically arranged heterophils, macrophages, and epithelioid macrophages, small to moderate numbers of lymphocytes and plasma cells, moderate numbers of multinucleated giant cells (foreign body type, 10-50 nuclei) and variable numbers of fibroblasts surround the adiaspores. At the center of some granulomas are degenerate adiaspore remnants admixed with heterophils or a core of necrotic debris. Granulomas multifocally extend up to or raise the visceral pleural surface. Adiaspore walls are intensely and stain intensely with periodic acid-Schiff stain.
Contributor Morphologic Diagnosis:
Pneumonia, granulomatous and heterophilic, chronic, multifocal to coalescing, severe; lungs, with adiaspores consistent with Emmonsia crescens.
Contributor Comment Adiaspiromycosis is associated with two causative agents: Emmonsia crescens and Emmonsia parva, which are saprophytic dimorphic fungi of the Ajellomycetaceae family. E. parva is distributed in small geographical areas in North America, Asia, Australia, and eastern Europe, has a small adiaspore diameter (10-20mm) and grows up to 40°C. E. crescens has a worldwide distribution, large adiaspores (up to 300mm), and grows up to 37°C.14
Following inhalation of conidia from soil, adiaspores develop and increase in size in host tissue which leads to granulomatous pneumonia in the host3. Small mammals such as rodents5,7 and mustelids9 are most commonly affected, but adiaspiromycosis has also been sporadically reported in larger mammals such as horses12, deer11, badgers10, foxes10 and dogs8. Adiaspiromycosis occurs rarely in humans and has a range of presentations from solitary pulmonary nodules to disseminated pulmonary disease and is occasionally fatal. A disseminated, extrapulmonary form has been described in immunosuppressed patients with acquired immunodeficiency syndrome and in one HIV-positive patient on immunosuppressive therapy following liver transplant.1. Adiaspiromycosis has been previously reported in cottontail rabbits (Sylvilagus audubonii) in New Mexico15 and is recognized in Oryctolagus cuniculus as a historic, occasional, pathogen of laboratory rabbits.
In this case, a wild European rabbit (Oryctolagus cuniculus) exhibited granulomatous pneumonia which was particularly marked both grossly and microscopically.6 All lung lobes were affected. Additionally, the cortex and medulla of the tracheobronchial lymph node were multifocally expanded by moderate numbers of adiaspores, with a similar histological appearance to those in the lungs, and surrounded by large numbers of epithelioid macrophages, moderate numbers of multinucleated giant cells (foreign body type, 10-50 nuclei) and fewer heterophils. Adiaspores do not replicate within host tissue and so the extent and severity of this case was most likely related to inhalation of large numbers of conidia. Adiaspore morphology was considered most consistent with E. crescens due to the diameter.
E. crescens infection can be confirmed by microdissection of adiaspores and PCR amplification using Emmonsia specific primers followed by DNA sequencing.2,12 In this case microdissection and PCR of formalin-fixed tissue was attempted using Emmonsia specific primers however no Emmonsia specific DNA was identified. This may have been due to the limitations of using formalin-fixed tissue for PCR amplification rather than fresh tissue. Fungal culture was not attempted, but culture of E. crescens is reported to be challenging and frequently unsuccessful.2
Recent phylogenetic studies have led to taxonomic revisions of E. parva and E. crescens. In one study, E. parva was shown to cluster in the Blastomyces genus (B. parvus)4, and other members of the Emmonsia genus with a yeast tissue form have been reclassified into the Emergomyces genus. It has, therefore, been proposed that Emmonsia crescens be assigned to the new genus Adiaspiromyces.4,6
Contributing Institution:
Department of Veterinary Medicine, The Queen's Veterinary School Hospital, University of Cambridge. Cambridge CB3 0ES, UK. https://www.vet.cam.ac.uk
JPC Diagnosis: Lung: Pneumonia, interstitial, granulomatous, diffuse, moderate, with numerous extracellular adiaspores.
JPC Comment: The contributor has submitted an excellent review of adiospiromycosis in animal species, from its pathogenesis to the nmost recent development in classification of this organism.
Historically,
the first descritpion of adispiromycosis as a pulmonary infection was first in
rabbits by Dr Chester Wilson Emmons (1900-1985) of the National Institutes of
Health. During his tenure at the NIH, he was the first medical mycologist at
the NIH as well as the head of the medical mycology section at the National
Institute for Allergic and Infectious Disease. Dr. Emmons designated the
fungus as Haplosporium parvum, but it was reexamined in 1959 by Cifrerri
and Montemartini, who renamed the organism Emmonsia parvum. Interestingly
(at least for this particular conference), Dr. C.W. Emmons, during his tenure
at NIH, is also credited for identifying the ecological niche for Cryptococcus
neoformans.
In 1960 Emmons himself added a second species, E. crescens, and proposed adiaspiromycosis as a more descriptive name for the disease. The name adiaspiromycosis is derived from Greek - ?a? for not or without, ?dia? ? by or through, and sporo, for seed - ultimately in reference to the fact that the spores to do replicate or disseminate within tissue. The severity of disease is largely the result of inoculum size and the ability of the host to mount an immune response.13
The first human case of adispiromycosis was reported in 1964, and most cases of
this relatively rare fungal infection have been reported to the result of E.
crescens (which has been reported in over 120 mammalians species as well.)
Most cases of E parva have been seen in immunosuppressed hosts. A
syndrome of granulomatous conjunctivitis in children in the Amazon basin has
been described with Emmonsia,13 although Rhinosporidium
could not be completely excluded.
Until recently, Emmonsia
was limited to two speceis, E. crescens and E. parvum. However, a
cluster of new Emmonsia-like fungi have been isolated in human patients
with atypical disseminated mycotic infections behaving more like more traditional
dimorphic fungi such as Blastomyces, highlighting the close relationship
of these two genera. (In fact, E parva is theorized to be closer
genetically to Blastomyces dermatitidis than to E. crescens. Seven
new species of Emmonsia have been identified since the 1990?s (usually
in individual immunosuppressed patients), with only one becoming a named
species (E. pasteuriana.) Newer species of Emmonsia also differ
from the historical members of the genus in that rather than exhibiting simple
increase in size, they possess the ability to convert to replicative yeasts and
disseminate to other tissues.13
References:
1. Anstead GM, Sutton DA, Graybill JR. Adiaspiromycosis causing respiratory failure and a review of human infections due to Emmonsia and Chrysosporium spp. J Clin Microbiol. 2012;50:1346?1354.
2. Borman AM, Simpson VR, et al. Adiaspiromycosis due to Emmonsia crescens is Widespread in Native British Mammals. Mycopathologia. 2009 Oct 16;168:153?163.
3. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer?s Pathology of Domestic Animals. 6th ed. Vol. 2. St. Louis, MO: Elsevier, 2016:584? 585.
4. Dukik K, Muñoz JF, Jiang Y, et al. Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales). Mycoses. 2017;60:296?309.
5. Fischer OA. Adiaspores of Emmonsia parva var. crescens in lungs of small rodents in a rural area. Acta Vet. Brno. 2001;70:345?352.
6. Hughes K, Borman AM. Adiaspiromycosis in a wild European rabbit, and a review of the literature. J Vet Diagnostic Investig. 2018; Published online May 2.
7. Kim, T; Han, J; Chang, S; et al. Adiaspiromycosis of an apodemus agrarius captured wild rodent in Korea. Lab Anim Res. 2012;28:67?69.
8. Koller LD, Patton NM, Whitsett DK. Adiaspiromycosis in the lungs of a dog. JAVMA. 1976 Dec 15;169:1316?1317.
9. Křivanec K, Otčená?ek M. Importance of free living mustelid carnivores in circulation of adiaspiromycosis. Mycopathologia. 1977;60:139?144.
10. Křivanec K, Otčená?ek M, ?lais J. Adiaspiromycosis in large free living carnivores. Mycopathologia. 1976;58:21?25.
11. Matsuda K, Niki H, Yukawa A, et al. First detection of adiaspiromycosis in the lungs of a deer. J Vet Med Sci. 2015;77:981?983.
12. Pusterla N, Pesavento PA., Leutenegger CM, et al. Disseminated pulmonary adiaspiromycosis caused by Emmonsia crescens in a horse. Equine Vet J. 2002;34:749?752.
13. Schwartz IS, Kenyon C, Feng P, Govender NP, Dukik, K, Sigler L, Jiang Y, Stielow JB, Munoz JF, Cuomo CA, Botha A, Stchigel AM, de Hoog GS. 50 years of Emmonsia dseease in humans: the dramatic emergence of a cluster of novel fungal pathogens. PLOS Pathogens 2015; 11(11): e1005198.
14. Sigler L. Ajellomyces crescens sp. nov., taxonomy of Emmonsia spp., and relatedness with Blastomyces dermatitidis (teleomorph Ajellomyces dermatitidis). J Med Vet Mycol. 1996;34:303?314.
15. Taylor RL, Miller BE, Rust JH. Adiaspiromycosis in small mammals of New Mexico. Mycologia. 2017;59:513?518.