Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 5
25 August 2019
CASE IV: 15-0463 (JPC 4119789)
Signalment: 9-month-old, male-entire, Rottweiler cross canine (Canis familiaris).
History: A deceased, male-entire, 9-month-old Rottweiler cross canine was submitted to the Murdoch University Anatomic Pathology diagnostic service for necropsy examination following barbiturate euthanasia. The dog had a progressive 5-week history of non-ambulatory bilateral hindlimb paresis progressing to paralysis and loss of deep pain; there was no history of prior trauma, clinical signs were non-responsive to carprofen. Otherwise the patient appeared healthy and well, and had been eating and drinking normally. At the time of euthanasia it had developed fecal and urinary incontinence and no pain was elicited on spinal palpation. A neurological examination carried out prior to euthanasia found the following:
· The panniculus reflex was absent caudal to L4 bilaterally, with a very sharp demarcation.
· Hind limbs: tonic-clonic patellar reflex bilaterally, cranial tibial clonic-tonic on left and intermittently tonic clonic on right. Withdrawal reflex bilaterally intact, sciatic unconvincingly present, absence of deep pain bilaterally on all digits. Negative Babinsky. Negative crossed extensor. Negative superficial sensation. No motor function exhibited.
· Anal tone: decreased to virtually absent.
The cadaver was unfortunately frozen and thawed prior to necropsy examination.
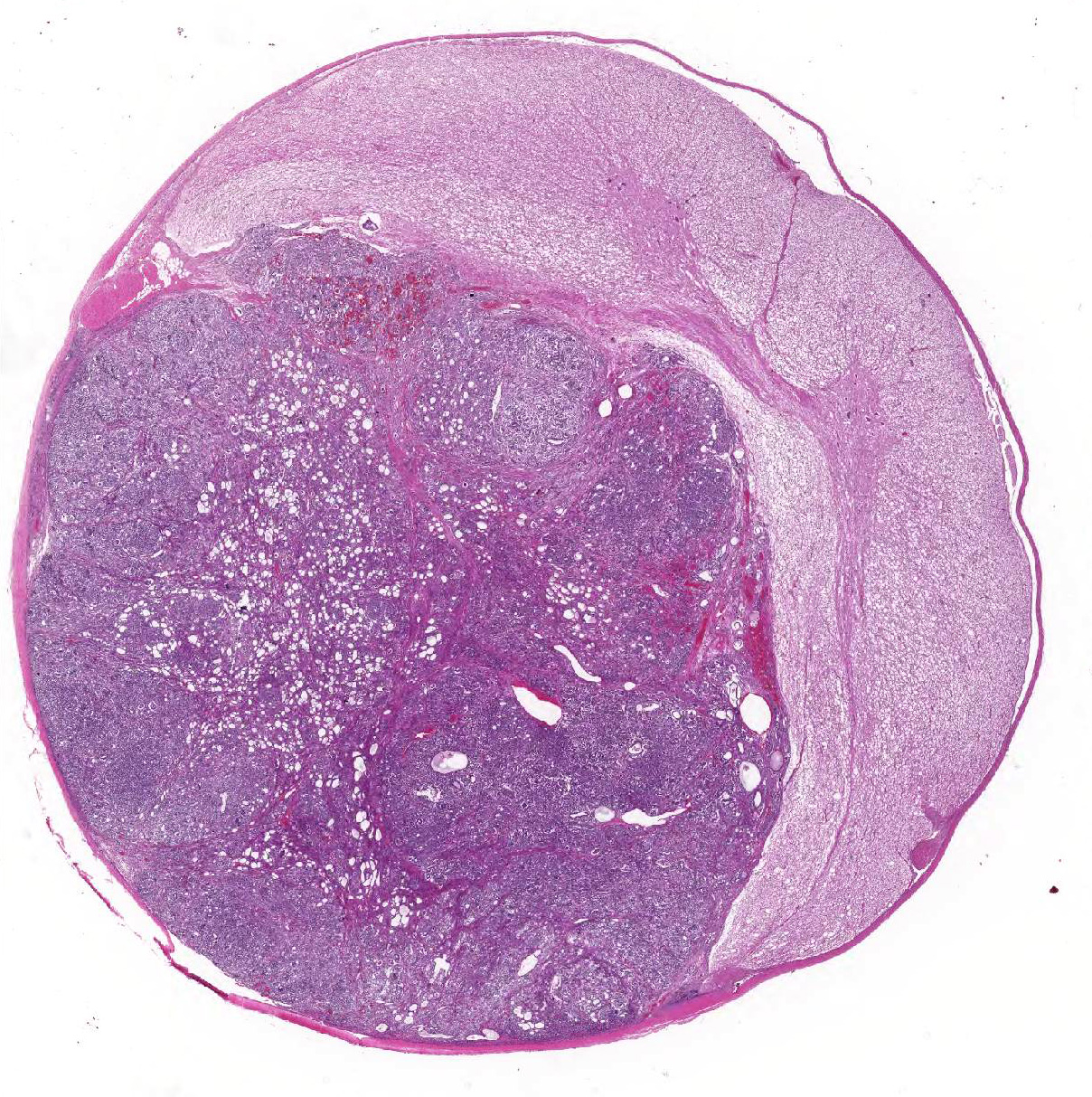
Gross Pathology: A focal, 10mm diameter, well-demarcated, mottled grey to pink-red nodule was identified within the spinal cord at the level of the T13/L1 intervertebral disc; its cut surface bulged slightly. Depending upon the level at which it was sectioned, the nodule was intradural and extramedullary, or intramedullary in location. The dorsal surfaces of the metatarsal regions bilaterally exhibited moderate, multifocal, chronic dermal erosions and ulcerations. The hindlimb musculature was mildly symmetrically atrophied.
Laboratory results: None performed.
Microscopic Description:
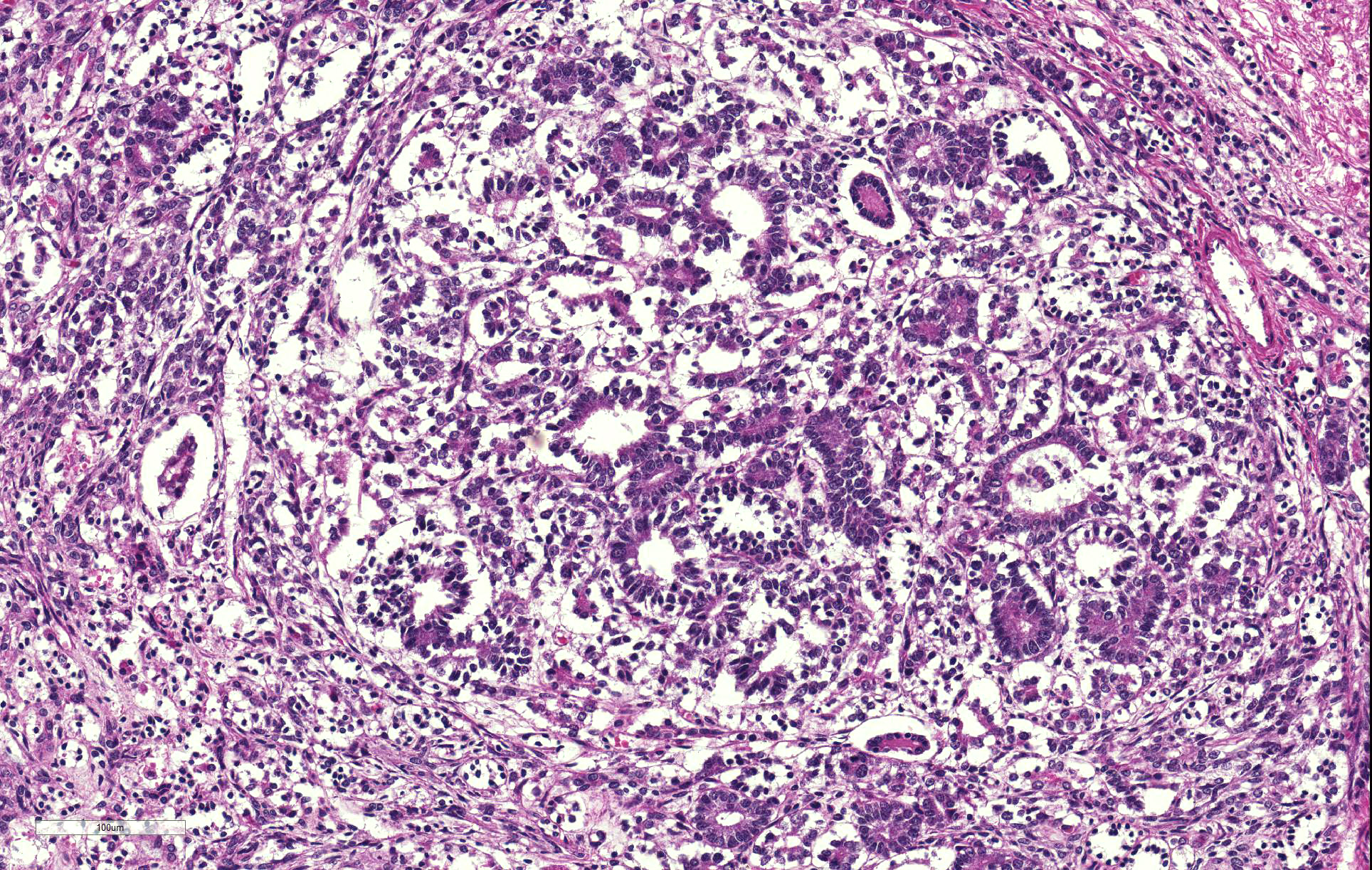
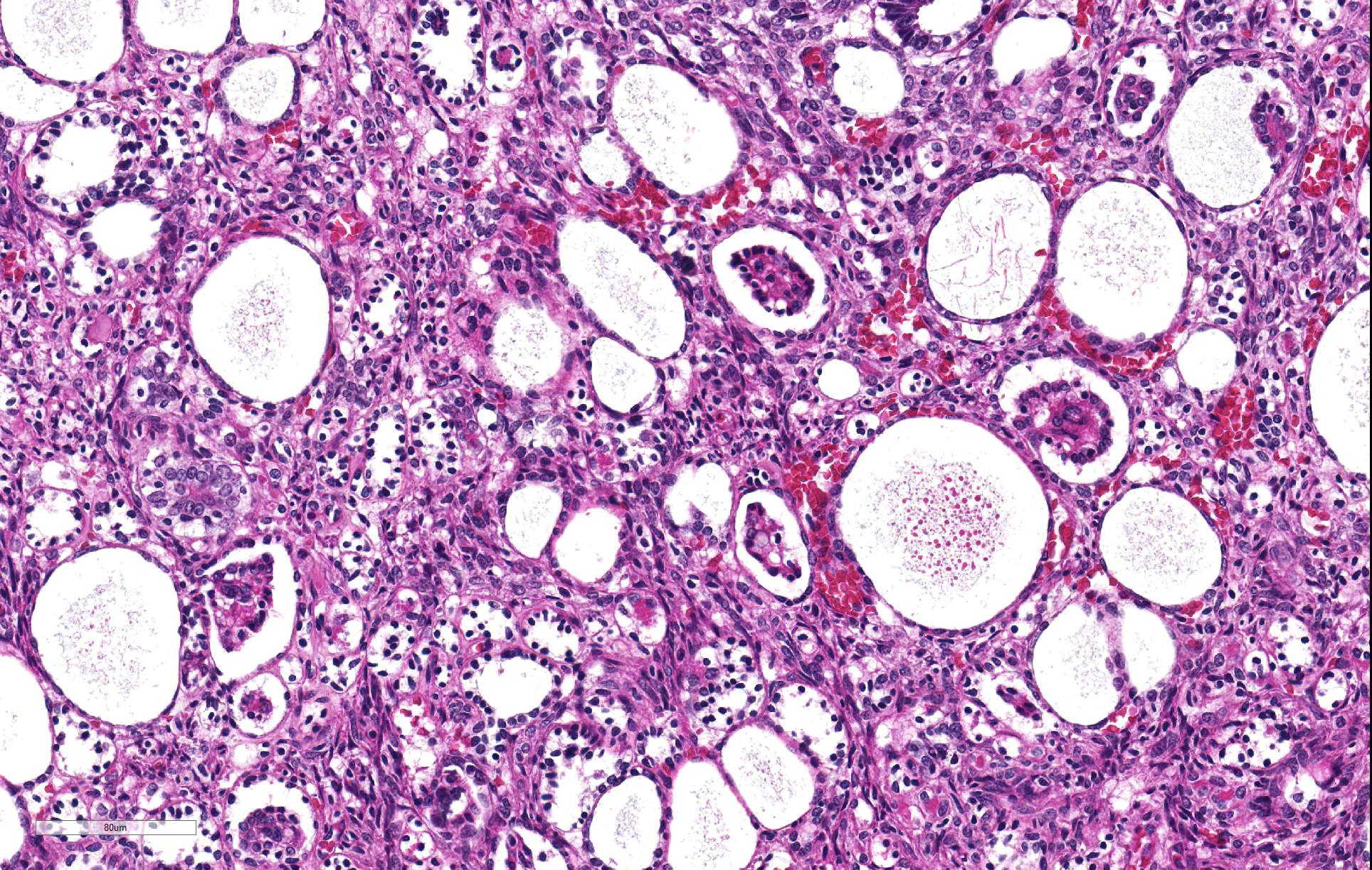
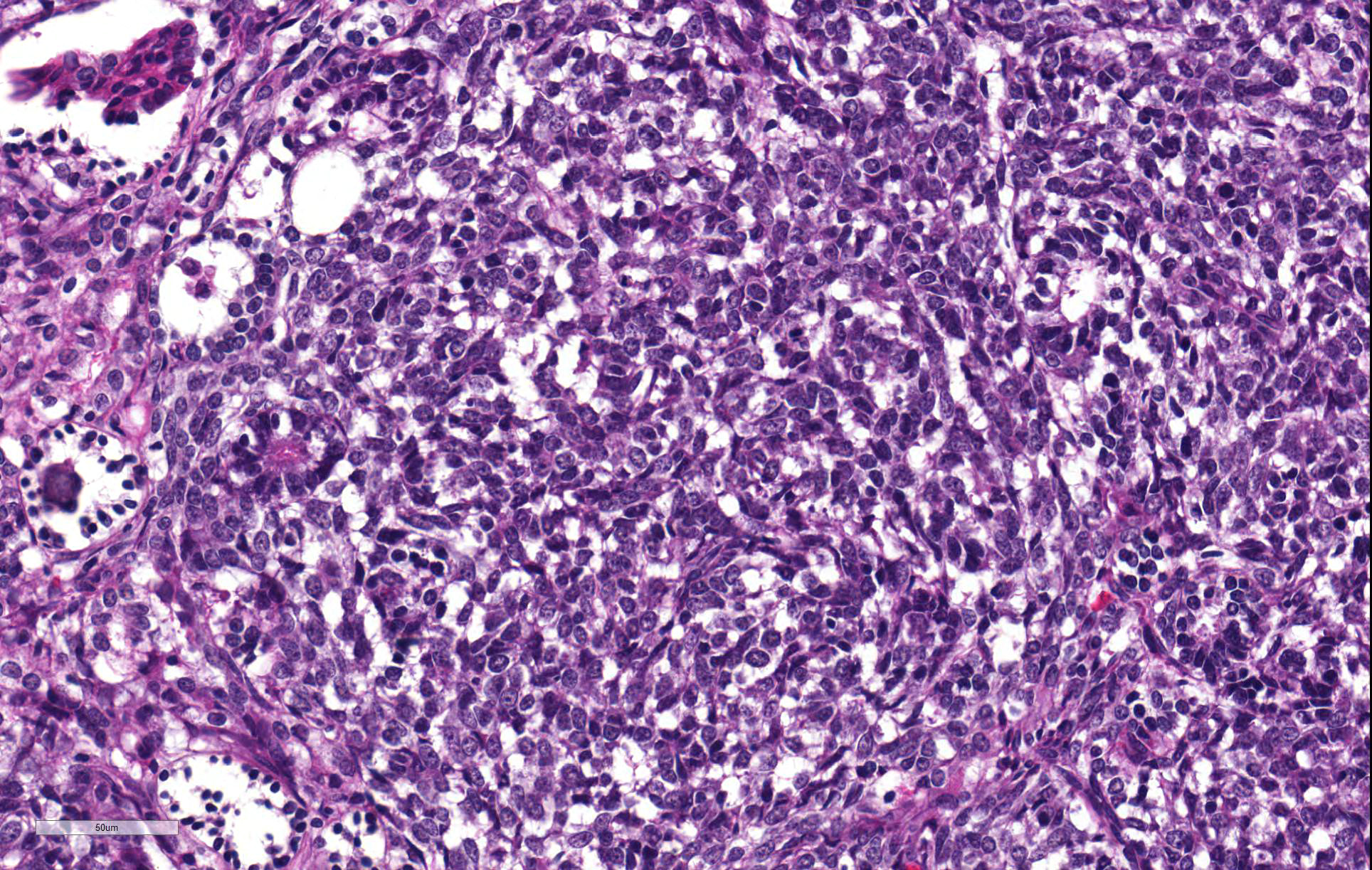
Thoracolumbar spinal cord: Effacing the grey matter, extending into the adjacent white matter and compressing the remaining adjacent spinal cord is a non-encapsulated, moderately well-demarcated, mildly infiltrative, moderately cellular neoplasm measuring 10mm in diameter. The neoplasm is composed of three distinct neoplastic cell populations. The first, epithelial, population forms tubules and acini lined by cuboidal, low columnar, to pseudostratified epithelium; frequently, the tubules form papillary projections (glomeruloid structures). 8 mitotic figures are seen in 10 HPF (400x) within this epithelial population. The second population consists of polygonal cells forming dense clumps or occasionally palisading along basement membranes; they possess indistinct cell borders, and a small amount of pink granular cytoplasm (primitive blastemal population). 12 mitotic figures are seen in 10 HPF (400x) within the blastemal population. The third and least abundant, mesenchymal, population is composed of spindloid cells, forming loose streams which ramify throughout the other two populations, and occasional whorls. Spindloid cell nuclei are round to ovoid with a single indistinct nucleolus and finely stippled to lacy chromatin; their cytoplasm is eosinophilic with indistinct cell borders. 5 mitotic figures are seen in 10 HPF (400x) within the mesenchymal population. Blood vessels within the neoplasm are distended with erythrocytes (hyperaemia). The remaining spinal cord parenchyma contains large numbers of variably sized clear spaces (rarefaction, freeze-thaw post-mortem artefact).
Contributor
Morphologic Diagnoses:
Spinal cord T13/L1: ectopic nephroblastoma.
Contributor Comment: Microscopic evaluation of the mass found within the spinal cord at the level of T13/L1 reveals a neoplastic infiltrate, which, given the lack of a renal lesion, is consistent with a primary spinal ectopic nephroblastoma.
Unlike primary renal nephroblastomas (Wilmsâ tumor), which are reported in a wide range of domestic companion and production animal species, including dogs, primary spinal ectopic nephroblastomas are rarely reported neoplasms that occur in young dogs between 5 months to 4 years, the median age being 14 months.1,4 Whilst they are rare, comprising merely approximately 1% of all canine primary CNS tumors, they are an important differential in the aforementioned age group.4 Of course, secondary spinal nephroblastomas can arise as a result of metastasis from a primary renal nephroblastoma; however the lungs and liver are more common secondary sites, with over 50% of canine cases of primary renal nephroblastoma exhibiting widespread pulmonary and hepatic metastases.6 Primary spinal ectopic nephroblastomas typically arise as a single mass, or sometimes multiple masses, at the level of the thoracolumbar junction between T10-L33,4 and exhibit both intramedullary and extramedullary intradural growth; extradural growth is also reported.1,11
Whilst historically German Shepherds and female dogs were thought to be predisposed1, a breed and sex predisposition is not supported in more recent reports, with dogs of variable sizes and breeds reported.3,4,11 Affected dogs exhibit clinical signs of a compressive myelopathy, i.e. progressive unilateral or bilateral paraparesis, paraplegia, and/or ataxia; the duration of clinical signs is reported to range from 2 to 60 days, with the median being 14 days.1,4 The prognosis appears variable, with one study involving 11 dogs having found the post-surgical resection median survival time to be 70.5 days;1 whilst another reported a median survival time of 374 days post surgery (6 dogs) or radiation (1 dog), and 55 days in 3 dogs only receiving palliative prednisone and gabapentin.5,11 The latter study also found that tumor location was important for prognostication â dogs with intramedullary nephroblastoma (n = 4) had a much shorter median survival time (140 days) compared to 380 days in the 6 dogs with intradural but extramedullary nephroblastoma;5 however, the 10 dogs in the study received varied treatment regimens. Previously it was not thought to metastasize,10 however multifocal spinal nephroblastoma is reported in 2 dogs, consistent with intraspinal metastasis through the cerebrospinal fluid.3,10 Metastasis to the caudal vena cava, adrenal glands, hepatic and mediastinal lymph nodes, pulmonary interstitium, bone marrow, and periosteum as well as extradural space of two vertebral bodies is reported in a single canine case with a dorsal retroperitoneal mass contiguous with a right renal tumor.2 However, it is difficult to ascertain whether the primary neoplasm was of renal or spinal origin in the reported case.
Their histogenic origin remains unconfirmed and controversial, given the complexity of nephrogenesis; it is believed they originate from (a) ectopic metanephric blastema or, (b) mesonephric rest tissue, either of which become entrapped between the dura mater and the spinal cord during embryogenesis.2,9 Nephrogenesis involves two embryologically distinct tissues - nephrogenic and ductogenic; the former developing from the intermediate mesoderm and progressing through the pronephros, mesonephros and metanephros phases.12 In humans, this progression normally ceases after 36 weeks gestation, whereupon the renal metanephric blastema disappear.12 Occasionally, the nephrogenic blastema fail to develop into normal mature renal parenchyma (however, it is not known whether arrest occurs at the mesonephros or metanephros phase in any given case8); persistent blastemal tissue is then referred to as nephrogenic rest tissue, which may undergo oncogenesis resulting in nephroblastoma12 (either primary renal in the case of renal nephrogenic rests, or primary extrarenal in the case of ectopic rests). In humans, extrarenal nephroblastomatosis, i.e. ectopic immature renal tissue, has been reported in such ectopic sites as the inguinal canal, testis, lumbosacral area, adrenal gland, thorax, colon, and heart;8,12 however, primary extrarenal nephroblastoma is rare, with few cases reported in the retroperitoneum and inguinal canal.8
Previously in the canine literature, this tumor was mistakenly thought to be of neuroectodermal origin, leading to its frequent misdiagnosis as one of the other main differentials for intradural spinal cord tumors in young dogs (i.e. ependymoma, medulloepithelioma, neuroepithelioma); it has also been erroneously classified as spinal cord blastoma, poorly differentiated astrocytoma, and hamartoma.4,5,10 Teratoma can be excluded due to the lack of other non-nephrogenic tissues within the neoplasm.12 As seen in this case, ectopic nephroblastomas are characterized by a triphasic neoplastic population, comprised of epithelial, blastemal, and stromal cells, redolent of Wilmsâ tumor.3,4,6 Distinctive histological features aiding diagnosis are the presence of tubules and acini, as well as glomerulus-like structures that resemble fetal glomeruli.3,4 Metaplastic differentiation towards muscle, bone or cartilage is frequently reported in renal nephroblastomas,6 however is not a feature of spinal ectopic nephroblastomas, with only 1 case reporting metaplastic cartilage formation.3 Wallerian degeneration (multifocal myelin sheath distension, swollen axons, and myelomacrophages) attributable to a compressive myelopathy is commonly seen; however it was felt in this case post-mortem change due to freeze-thaw artefact obscured ability to observe such changes.
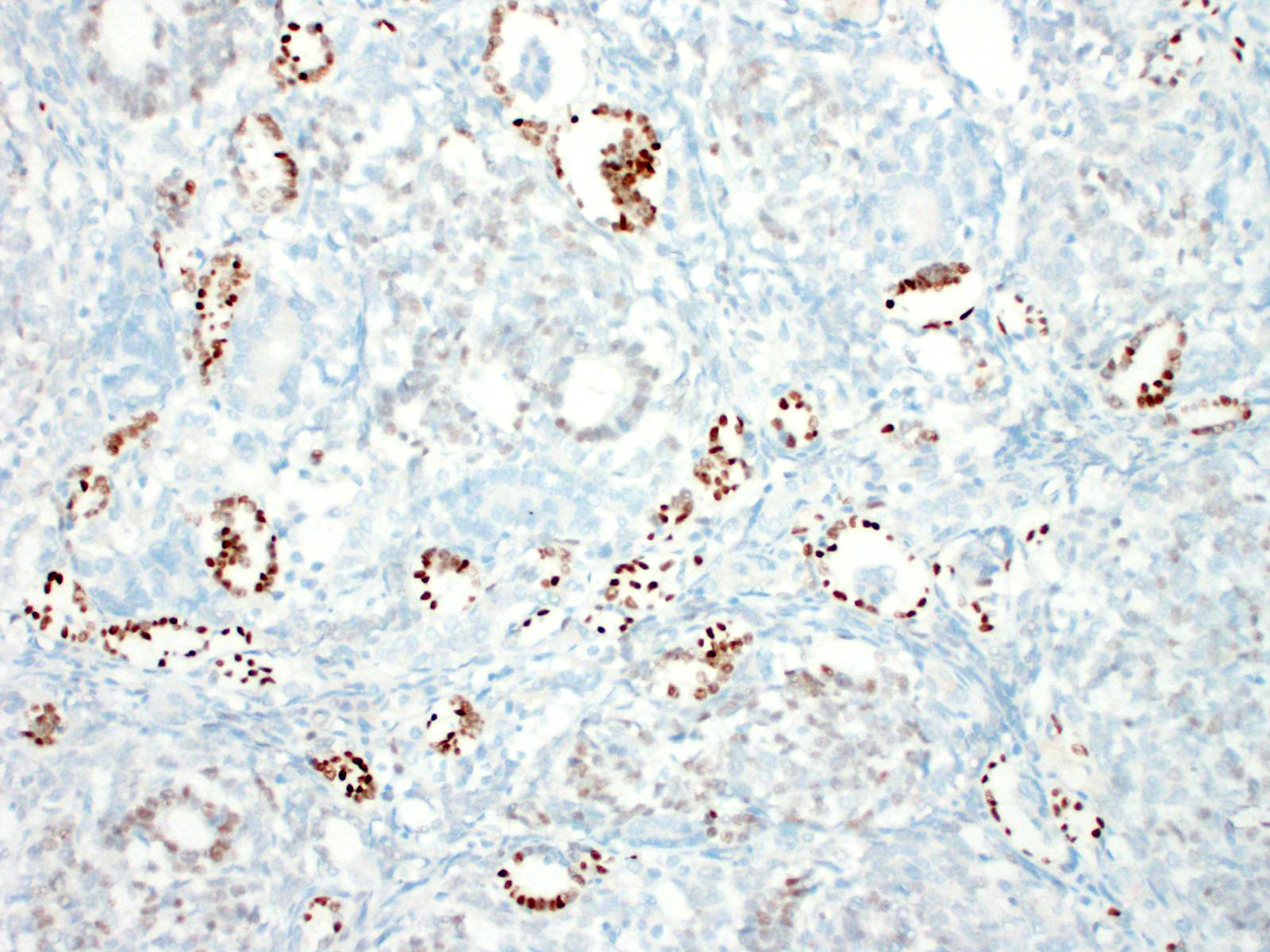
Immunohistochemistry can aid diagnosis, and also supports their ectopic renal origin.10 The blastemal and mesenchymal (stromal) populations are immunoreactive for vimentin, whilst the epithelial population forming tubules/acini shows immunoreactivity for cytokeratin.10 Immunoreactivity of polysialic acid to the blastemal nuclei is seen;4,10 however the definitive antigenic marker is Wilmsâ tumor gene product (WT-1) â the blastemal nuclei in the glomerulus-like structures are typically strongly immunopositive.4 There are, however, reports of canine cases that are negative for this marker; presumably due to abnormal antigen expression by tumour cells.1,6 In human nephroblastoma, a poorer prognosis is seen in cases in which stronger blastemal WT-1 expression is observed,1 however this correlation has not been found in canines. Primary spinal ectopic nephroblastomas lack immunoreactivity with routine neuronal (SYN, NeuN, and TNF) and glial (Olig2, GFAP) markers.4 Nephroblastomas lack immunoreactivity for GFAP and neurofilaments, yet their epithelial population is immunopositive for cytokeratin, which rules out the main differential diagnoses, the primitive neuroectodermal tumors;4 in particular, ependymomas, given the perivascular pseudorosettes and true rosettes typical of ependymomas are similar to the tubules found in nephroblastomas.10
Contributing Institution:
Veterinary Pathology Department
School of Veterinary and Life Sciences
Murdoch University
90 South Street
Murdoch, Western Australia
6150, Australia
http://www.murdoch.edu.au/School-of-Veterinary-and-Life-Sciences/
JPC Diagnosis: Spinal cord: Ectopic nephroblastoma (spinal nephroblastoma)
JPC Comment: The contributor has done an excellent job summarizing this uncommon and very unique tumor of young dogs.
The Wilms tumor antigen is a tumor suppressor gene that it humans is found at 11p13. It functions as a regulator of transcription in the inner layer of intermediate mesoderm and inhibits transcription of several growth-promoting genes, and plays a critical role in the development of the genitourinary tract, spleen and mesothelium. First identified as a tumor suppressor gene in association with Wilmâs tumors in humans, the name WT-1 would lead one to believe that the immunohistochemical stain targeting this gene product would be specific for Wilmâs tumor. However, it is also present in a number of normal tissues, including CD-34 positive stem cells, glomerular podocytes and mesangial cells, Sertoli and granulosa cells, ovarian stroma and surface epithelium, uterine endometrial stroma and myometrium, and mesothelium.7 It is expressed in a wide range of neoplasms in humans, including nephroblastoma, mesothelioma, metanephric adenoma, ovarian carcinoma, transitional cell carcinomas, among others.7
While nephroblastomas have been rarely identified as intra- and extradural masses in humans, it has almost always been a consequence of metastasis (10% of Wilmâs tumor present with metastatic foci), and less commonly invasive growth through nerve root foramina. Development from rests of ectopic nephrogenic tissue has not yet been documented. Contrarily, there appears to be only one report of spinal nephroblastoma in the dog attributable to metastasis of a renal primary, as opposed to growth of ectopic metanephric blastema.9
References:
1. Brewer DM., Cerda-Gonzalez, S., Dewey, CW., Diep, AN., Van Horne, K., McDonough, SP. Spinal cord nephroblastoma in dogs: 11 cases (1985-2007). JAVMA. 2011; 238(5): 618-624.
2. Gasser, AM., Bush, WW., Smith, S., Walton, R. Extradural spinal, bone marrow, and renal nephroblastoma. J Am Anim Hosp Assoc. 2003; 39: 80â85.
3. Henker, LC., Bianchi, RM., Vargas, TP., de Oliveira, EC., Driemeier, D., Pavarini, SP. Multifocal spinal cord nephroblastoma in a dog. J Comp Path. 2018; 158: 12-16.
4. Higgins, RJ., Bollen, AW., Dickinson, PJ., Sisó-Llonch. S. Tumors of the Nervous System. In: Meuten DJ, ed. Tumors in Domestic Animals: 5th ed. Ames, IA: John Wiley & Sons Inc.; 2017: 863-864.
5. Liebel, F-X., Rossmeisel, JH., Lanz, O., Robertson, JL. Vet Surg. 2011; 40: 244-252.
6. Meuten, DJ., Meuten, TLK. Tumours of the urinary system. In: Meuten DJ, ed. Tumors in Domestic Animals: 5th ed. Ames, IA: John Wiley & Sons Inc.; 2017: 646-650.
7. Nakatsuka S, Oju Y Horiuchi T et al. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod Pathol 2006; 19:804-814.
8. Oottamasathien, S., Wills, ML., Brock III, JW., Pope IV, JC. Primary extrarenal nephroblastomatosis. Urology. 2007; 184.e3-184.e4.
9. Petit A, Rubo A Durand C, Piolat C, Perret, Cecile P, Pagnier A, Plantaz, D, Sartelet H. A Wilmsâ tumor with spinal cord compression: An extrarenal origin?
10. Terrell, SP., Platt, SR., Chrisman, CL., Homer, BL., de Lahunta, A., Summers, BA. Possible intraspinal metastasis of a canine spinal cord nephroblastoma. Vet Pathol. 2000; 37: 94-97.
11. Traslavina, RP., Aleman, M., Affolter, VK., LeCouteur, RA., Ramsamooj, R., Higgins, RJ. Pathology in practice. Spinal cord (ectopic) nephroblastoma in a dog. J Am Vet Med Assoc. 2013; 242(12): 1661-1663.
12. Wu, Y., Zhu, X., Wang, X., Wang, H., Cao, X., Wang, J. Extrarenal nephroblastomatosis in children: a report of two cases. BMC Pediatrics. 2014; 14: 255-259.