Signalment:
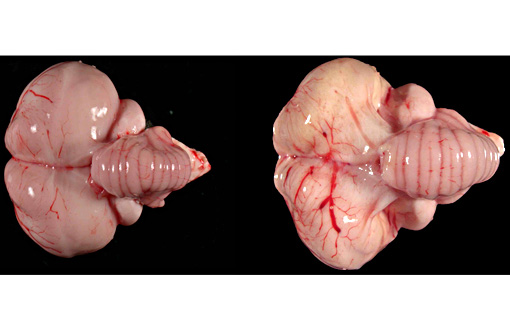
Gross Description:
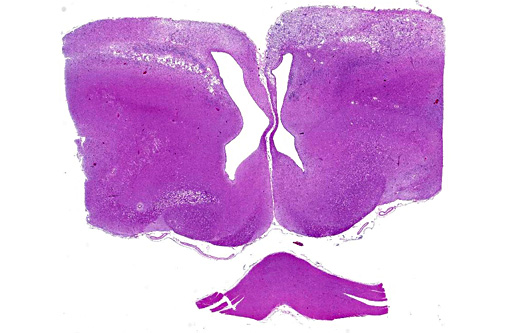
Histopathologic Description:
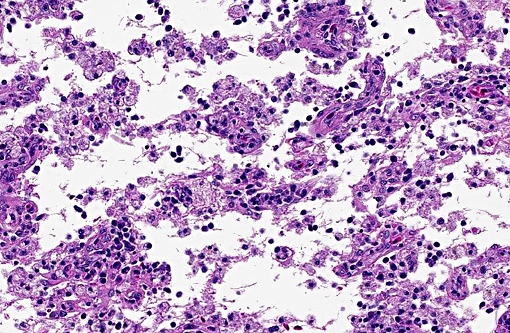
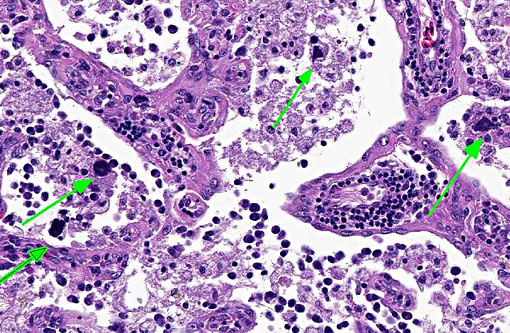
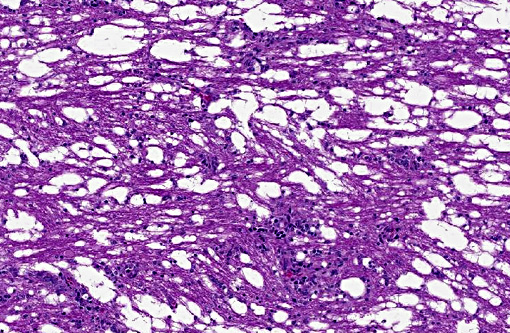
There was bilateral symmetric pan-necrosis of the gray matter of the dorsal aspect of the cerebral hemispheres (pallium) along the lateral ventricles. The neuroparenchyma was collapsed and cavitated around what appeared to be a remaining scaffold of vasculature. The neuroparenchyma was largely infiltrated and replaced by numerous macrophages with gitter cell morphology in the pallium. In addition, there was a widespread massive lymphoplasmacytic perivascular infiltration. The endothelial cells were hypertrophied. Numerous cells contained basophilic granular material (interpreted to be calcified mitochondria) and occasional neurons were entirely calcified.
The gray matter adjacent and subjacent to the necrotic parenchyma was hypercellular. The hypercellularity was due to infiltration with lymphocytes and macrophages and due to infiltration by reactive astrocytes. These astrocytes were plump, had an increased amount of a faint eosinophilic cytoplasm and one and occasionally two enlarged vacuolar (euchromatic) nuclei. Occasionally distinctly eosinophilic globules were present in the gray matter (interpreted to be spheroids).
White matter tracts such as the occipitomesencephalic tracts and optic tracts had a bilateral symmetric spongiform change characterized by the presence of numerous optically empty spaces (interpreted as myelin sheath edema). In addition, mild to moderate infiltrates of lymphocytes and plasma cells were present around capillaries of the white matter.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Gross lesions of WN disease in bald eagles may include macroscopically appreciable cerebral pan-necrosis as in the presented case in approximately half the cases and in individual cases may include myocarditis and endophthalmitis.(10) The presence of a triad of histopathological changes including encephalitis, endophthalmitis, and myocarditis are a hallmark of WN disease in bald eagles and other Accipitridae including Coopers hawks (Accipiter cooperi), red tailed hawks (Buteo jamaicensis), and goshawks (Accipiter gentilis).(5,8-10) The pathogenesis of the cerebral pan-necrosis may include a vasogenic component due to damage of endothelial cells by the virus during an early phase of the infection or due to damage of the capillary integrity in the wake of the inflammatory response by extravasating inflammatory cells. Overt WNV-associated arterial necrosis has been described in kestrels and other falcons but does not appear to be a prominent feature of WN disease in hawks and eagles.(4,12) Alternatively or concurrently, the pathogenesis of the pan-necrosis may include direct cytolysis by the virus targeting neurons and possibly glial cells and/or cytolysis of neurons and glial cells as a result of bystander injury in the context of the antiviral immune response. High WNV antigen concentrations are present in the cerebrum (and cerebellum) of bald eagles with fairly acute WNV-associated encephalitis.(10) The cerebral necrosis was similar to a lesion described in one pigeon that was experimentally infected with highly pathogenic avian influenza virus.(2)
Based on immunohistochemical analysis, brain (cerebrum and cerebellum), eyes (retina) and to a lesser extent heart and kidney harbor viral antigen similar to findings in other Accipitridae. PCR of brain tissue commonly yields a positive result in bald eagles with West Nile disease except for cases that had a significantly prolonged disease, e.g. because they were kept and cared for at a rehabilitation facility for months. In these animals, detection of WNV-specific antibodies in the CSF may be helpful to confirm WN encephalitis.(10)
JPC Diagnosis:
Conference Comment:
WNV has a wide range of tissue tropism, thus there are no pathognomonic macroscopic lesions. Multiorgan hemorrhages are most characteristically found, and specifically to raptors, cerebral atrophy and malacia is often observed. Microscopically, lymphoplasmacytic and histiocytic inflammation, necrosis, and hemorrhage occur most commonly in the CNS, heart, kidney, spleen and liver. Paradoxically, the most susceptible species have the least amount of inflammation, and raptors which contract more chronic disease develop the triad of lesions described by the contributor.(1)
WNV contains two envelope glycoproteins, E1 and E2, which facilitate tropism for specific tissues.(11) Neuroinvasion requires crossing the blood-brain barrier and may be assisted by the release of proinflammatory cytokines.(11) The chemoreceptor CCR5 contributes to neuroinvasive resistance in humans, as those with CCR5 mutations have an increased rate of symptomatic infection, though this link has not been identified in animals.(3)
References:
1. Gamino V, H+�-�fle U. Pathology and tissue tropism of natural West Nile virus infection in birds: a review. Vet Res 2013;44:39.
2. Klopfleisch R, Werner O, Mundt E, et al. Neurotropism of highly pathogenic avian influenza virus A/Chicken/Indonesia/2003 (H5N1) in experimentally infected pigeons (Columbia livia f. domestica). Vet Pathol. 2006;43: 463-470.
3. McAdam AJ, Milner DA, Sharpe AH. Infectious disease. In: Kumar V, Abbas AK, Aster JC, eds. Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier Saunders; 2015:356-357.
4. Nemeth N, Gould D, Bowen R, Komar N. Natural and experimental West Nile virus infection in five raptor species. J Wildl Dis. 2006;42:113.
5. Pauli AM, Cruz-Martinez LA, Ponder J et al. Ophthalmologic and oculopathologic findings in red tailed hawks and Coopers hawks with naturally acquired West Nile virus infection. J Am Vet Med Assoc. 2007;231(8):1240-1248.
6. Pierson TC, Diamond MS. Flaviviruses. In: Knipe DM, Howley PM, Cohen JI, et al. eds. Fields virology, 6th ed. Philadelphia, PA: Lippincott, Williams & Wilkins, 2013: 760-762.Â
7. W+�-+nschmann A, Rejmanek D, Conrad PA, et al.: Natural Sarcocystis falcatula infections in free ranging eagles in North America. J Vet Diagn Invest 2010; 22: 282-289.
8. W+�-+nschmann A, Shivers J, Bender J, et al. Pathologic findings in red-tailed hawks (Buteo jamaicensis) and Coopers hawks (Accipiter cooperi) naturally infected with West Nile virus. Avian Dis. 2004;48:570580.
9. W+�-+nschmann A, Shivers J, Bender J, et al. Pathologic and immunohistochemical findings in goshawks (Accipiter gentilis) and great horned owls (Bubo virginianus) naturally infected with West Nile virus. Avian Dis. 2005;49:252259.
10. W+�-+nschmann A, Timurkaan N, Armien AGA, et al. Clinical, pathological, and immunohistochemical findings in bald eagles (Haliaeetus leucocephalus) and golden eagles (Aquila chysaetos) naturally infected with West Nile virus. J Vet Diagn Invest. accepted for publication.
11. Zachary JF. Mechanisms of microbial infections. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier Mosby; 2012:227-228.
12. Ziegler U, Angenvoort J, Fischer D, et al. Pathogenesis of West Nile virus lineage 1 and 2 in experimentally infected large falcons. Vet Microbiol. 2013;161:263-273.