CASE I: N17-663 (JPC 4117535)
Signalment:
13-year-old, male, neutered, American Eskimo dog, (Canis lupus familiaris)
History:
This dog presented for acute onset of seizures. Magnetic resonance imaging showed a right-sided forebrain mass with associated hemorrhage. Due to poor prognosis and clinical deterioration, the dog was euthanized.
Gross Pathology:
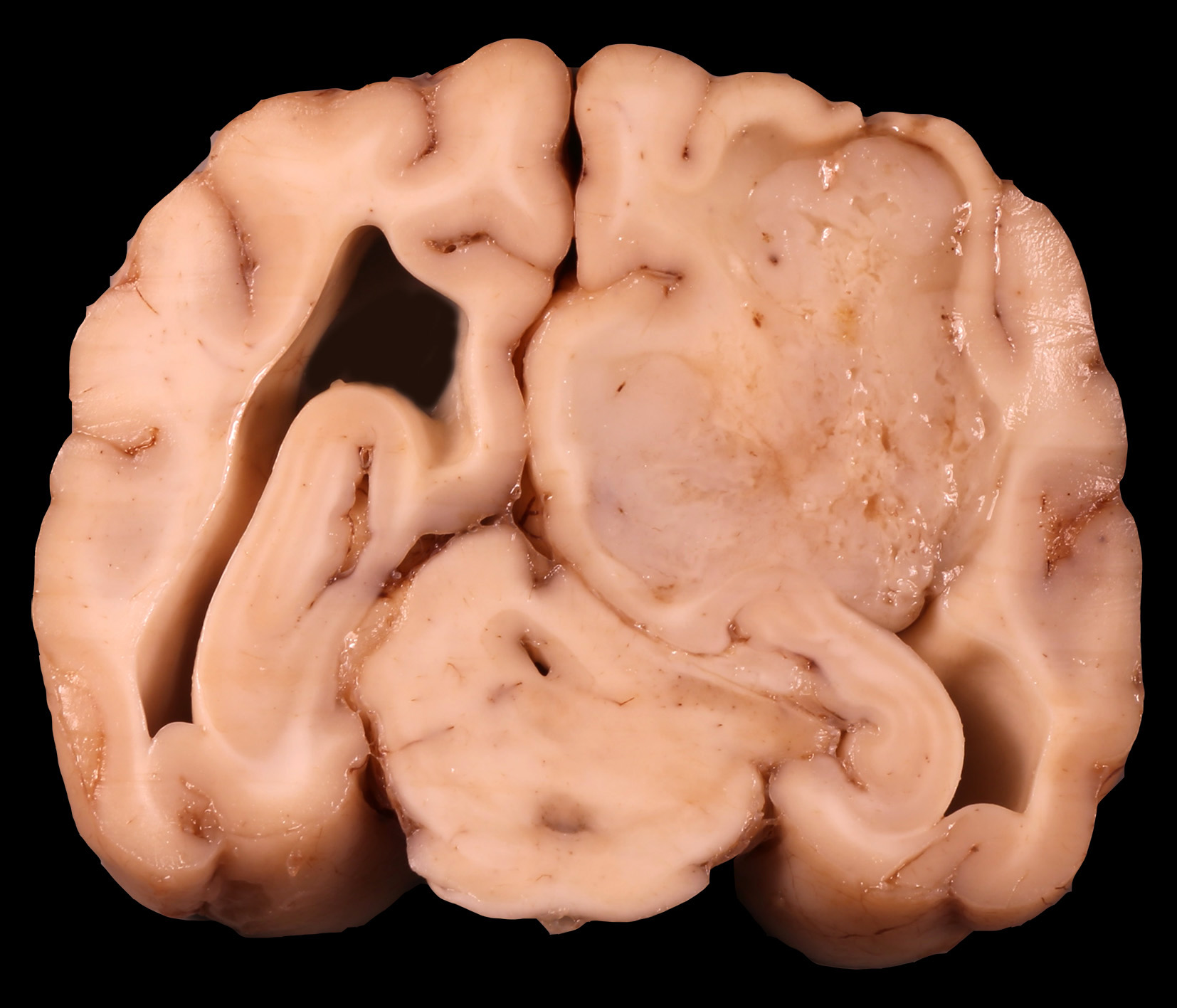
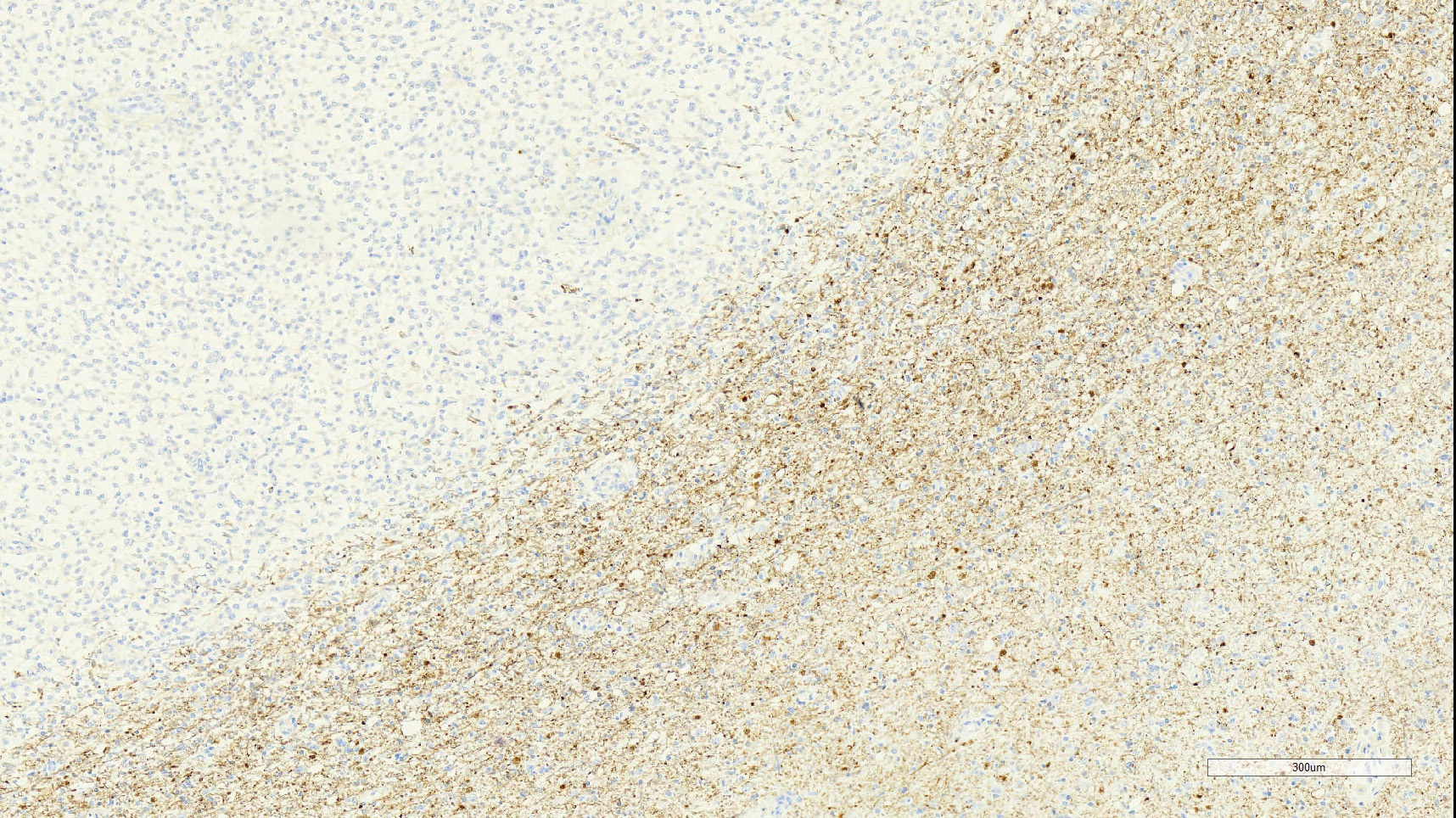
Grossly the right cerebral hemisphere was expanded by a 3 x 3 x 2.5 cm, poorly circumscribed, irregular, multilobulated, white to tan, firm to gelatinous, multifocally hemorrhagic mass that projected into the right lateral ventricle and compressed the thalamus.
Laboratory results:
No laboratory findings reported.
Microscopic Description:
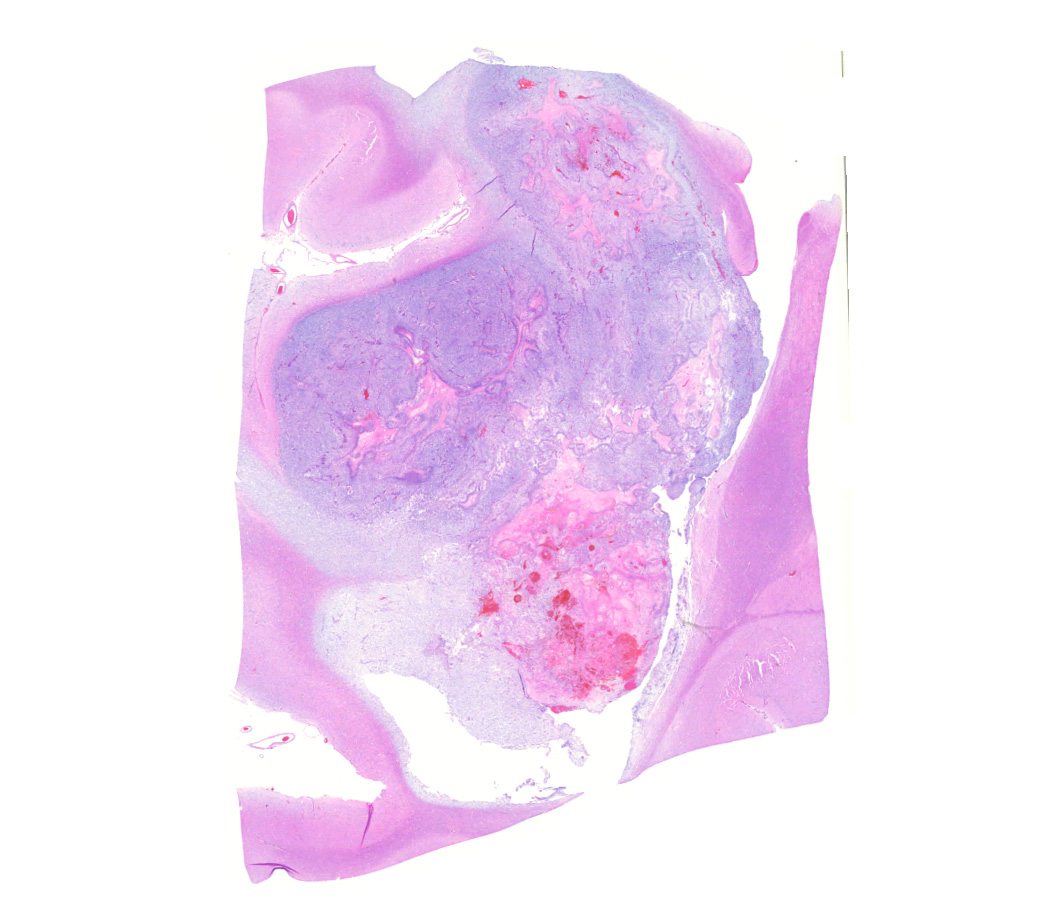
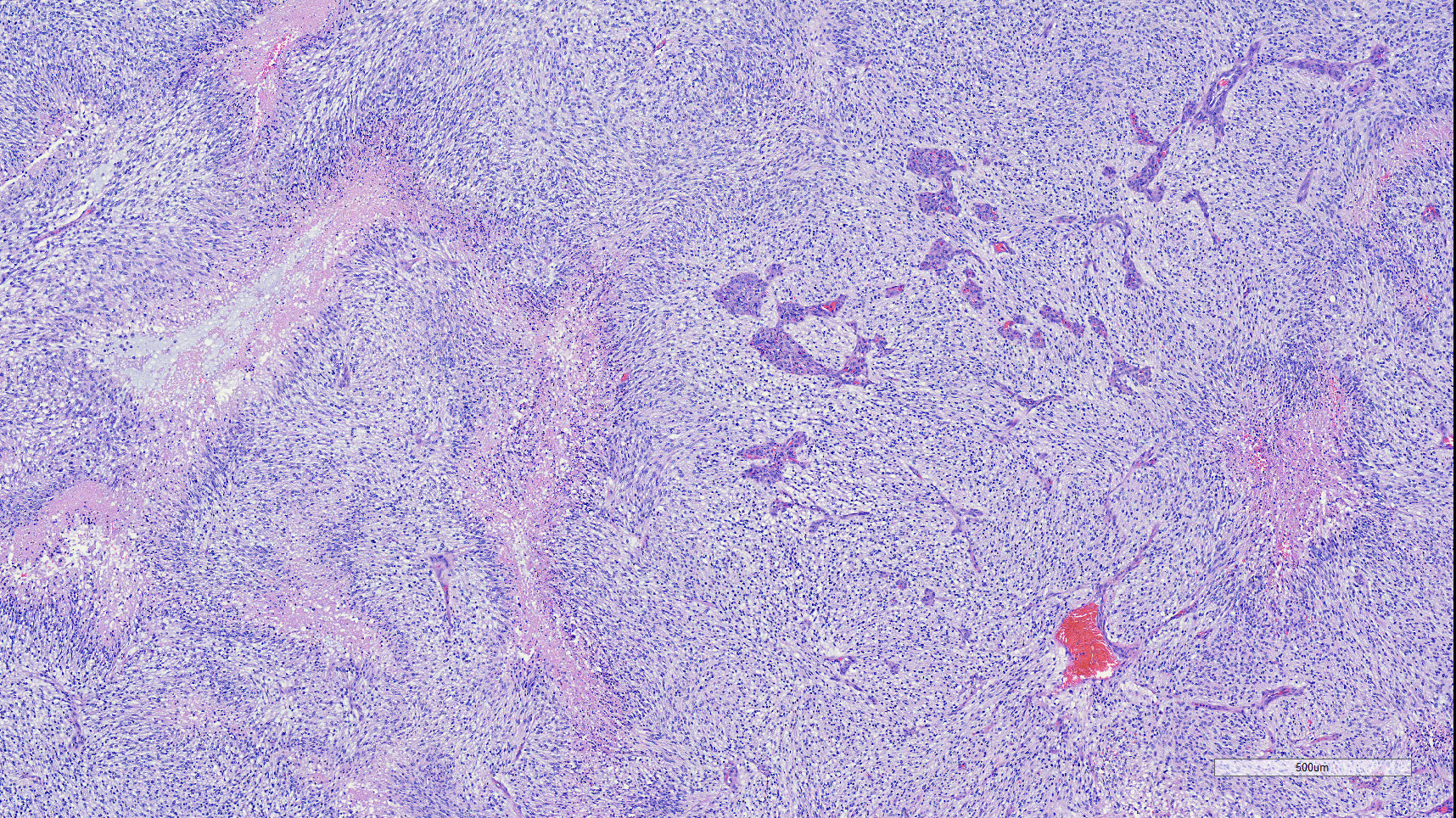
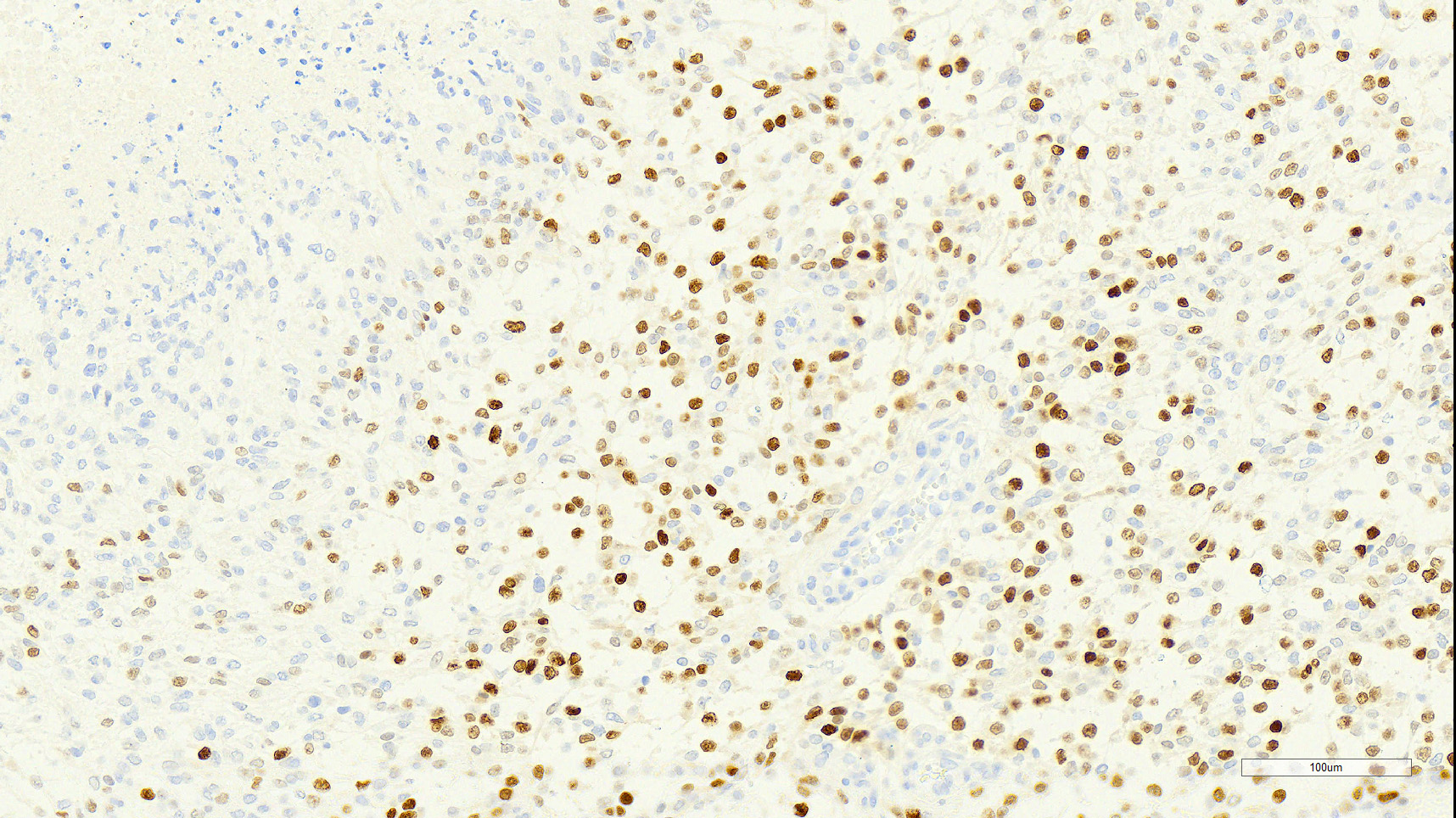
Brain: Effacing and infiltrating the neuropil of the cerebral cortex and spilling into the lateral ventricle, is a poorly demarcated, unencapsulated, densely cellular neoplasm composed of spindle to stellate cells arranged in disorganized streams and bundles that are often arranged perpendicularly (pseudo-palisading) along extensive serpiginous areas of coagulative to liquefactive necrosis that contain abundant karyorrhectic and cellular debris mixed with fibrin and hemorrhage. Neoplastic cells have indistinct cell borders, with scant to moderate amounts of eosinophilic cytoplasm. Nuclei are round to oval, with finely stippled to vesicular chromatin, and 1-3 small nucleoli. Anisocytosis and anisokaryosis are moderate. Multifocally, neoplastic cells are arranged in dense sheets supported by a myxoid matrix, have variably distinct cell borders, moderate amounts of vacuolated cytoplasm, and round to oval, uniform nuclei. There are 24 mitotic figures in 10 high power fields. Throughout the neoplasm, there is proliferation of blood vessels with hypertrophied endothelium that frequently form glomeruloid structures.
Contributor's Morphologic Diagnoses:
Brain: astrocytoma,
grade IV (glioblastoma)
Contributor's Comment:
Astrocytomas account for approximately 10-13% of primary central nervous system tumors in dogs. 7, 12, 14 The incidence is higher in brachycephalic breeds (approximately 23 times), but they can occur in any breed. 2, 4, 15 Incidence of astrocytic neoplasia in dogs, as in humans, increases with age.15 Astro-cytomas are a diverse group of gliomas that in dogs are subdivided in two distinct subgroups based on their growth patterns: infiltrative or diffuse, and well-demarcated.4 Diffuse astrocytic tumors are classified into four grades (I to IV), with grade IV being the most malignant form.2, 4, 12
Glioblastoma (grade IV astrocytoma) has been reported to represent 0.5 to 3% of all primary central nervous tumors in the dog.3,13, 14 Glioblastoma is the most common human brain tumor, and comprises 12-15% of all primary central nervous system tumors and 50-60% of all astrocytomas.7
Canine glioblastomas, like their human counterparts, are highly invasive. The infil-trative path within the normal brain parenchyma is not random and often follows white matter tracts, perivascular spaces and the subependyma.15
In humans, glioblastomas can be primary (de novo) tumors or secondary tumors that de-velop from a preexisting glial tumor, either diffuse astrocytomas (WHO grade II) or anaplastic astrocytomas (WHO grade III).15 In veterinary species, there is no evidence that glioblastomas arise from preexisting benign astrocytic/glial tumors.5
The most recent update to the WHO classification of Tumors of the Central Nervous System in humans includes molecular parameters (particularly genotype) in addition to histology to define and characterize many tumors.1,8,9 Diffuse infiltrative astrocytomas of adults are now subdivided according to the mutational status of isocitrate dehydrogenase-1 or -2 (IDH1/2) genes into IDH-mutant or IDH-wildtype 1, 9, with the large majority of IDH-wildtype astrocytomas (90-95%) being primary glioblastomas (WHO grade IV). These mutations have not been found in canine gliomas.5, 11
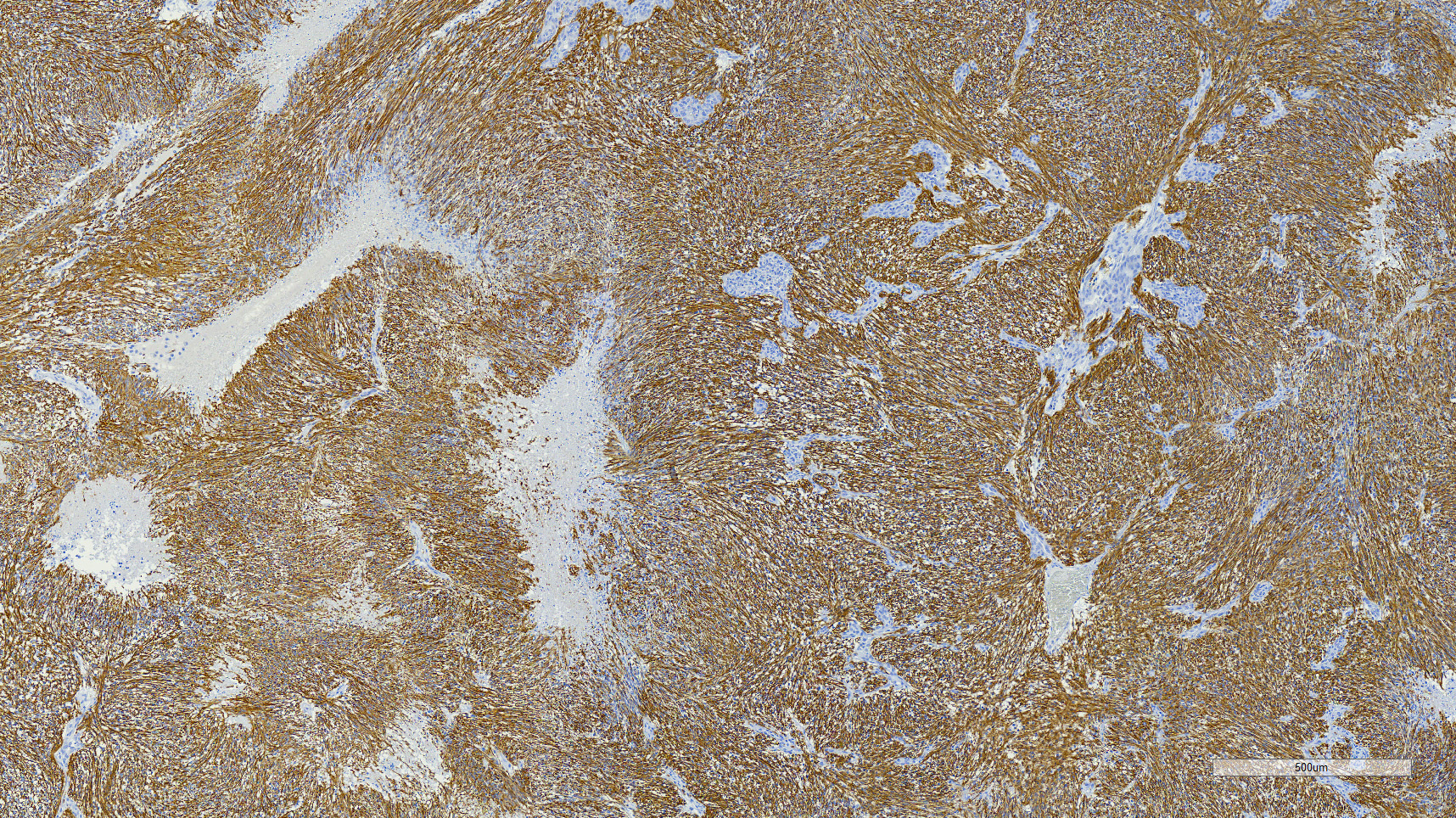
The main criteria for histological classification and grading of astrocytomas are the cell density, nuclear atypia and mitotic count. Astrocytomas consistently express glial fibrillary acidic protein (GFAP) immuno-reactivity, which decreases in the higher grades 4 and is the feature that suggests the glial origin of these tumors.15 In this dog, most neoplastic cells (>90%) exhibited strong cytoplasmic immunoreactivity for GFAP. Olig2 nuclear immunoreactivity was observed in a large population of the neoplastic cells (approximately 60%). Although this has been used as a marker for cells of oligodendroglial origin, astrocytomas in dogs and cats frequently express Olig2.5,10, 16 Lack of expression of CNPase by the neoplastic cells makes oligodendroglial origin or differentiation less likely and further supports an astrocytic origin.
Additional criteria for diagnosis of grade IV astrocytoma must include at least one of two essential diagnostic features: necrosis with marked glial pseudo-palisading and/or microvascular proliferation.4 Both of these features were quite prominent in this case.
Canine glioblastomas overexpress EGFR, PDGFR-?, and insulin-like growth factor binding protein 2 (IGFBP-2).2,3,15,16 Add-itionally, greater expression of VEGF, VEGFR-1, VEGFR-2, EGFR-1, IL-13RA2 has been described in canine glioblastomas compared to other intracranial brain tumors. 3,7,15 Microvascular proliferation is pre-sumably driven, in part, by this increased expression of VEGF.7 Proliferative index, measured by Ki67 expression, is described in the literature for glioblastomas and other glial tumors3,7,16, although its significance or prognostic value has yet to be determined in veterinary species.
Contributing Institution:
Cummings School of Veterinary Medicine at Tufts University
Department of Biomedical Sciences, Section of Pathology
200 Westboro Rd.
North Grafton, MA. 01536
http://vet.tufts.edu/department-of-biomedical-sciences/research/pathology/
JPC Diagnosis:
Cerebrum: Astrocytoma, high grade (previously known as astrocytoma grade IV or glioblastoma).
JPC Comment:
The contributor provides an outstanding review of the criteria used for the diagnosis of a grade IV astrocytoma. Since 1999, diagnostic criteria for canine glial tumors have been derived from the World Health Organization (WHO) Tumor Fascicle Histologic Classification of Tumors of the Nervous System of Domestic Animals, in addition to immunohistochemical markers of glial populations, which have become more readily available.6
Following the submission of this case to the WSC, the National Cancer Institute-led multidisciplinary Comparative Brain Tumor Consortium (CBTC) convened a glioma pathology board, composed of both veterinary and physician neuropathologists with the immediate goal of improving existing glioma classification methods, which would in turn would support harmonization of phenotypic characterization between species, serving to bridge veterinary and physician neuropathology and strengthen the validity of the dog as a naturally occurring, translationally relevant model of human glioma. One of several outcomes of the consortium was the creation of a new classification scheme to promote diagnostic consistency across veterinary institutions and to harmonize future comparative research and hypothesis-based investigations designed to aid in clinical management of canine glioma patients, especially the addition of future molecular markers that may stratify canine patients as is used for human patients with gliomas.6
Gliomas under the new grading scheme are categorized as an oligodendroglioma, astrocytoma, or undefined glioma. To be classified as an oligodendroglioma >80% of the tumor must meet the one or more of the following criteria: Round nuclei with a coarse chromatin pattern, scant to moderate eosinophilic or lost cytoplasm (artifact), pseudorosettes, secondary structures, nuclear rowing, myxoid to mucinous matrix (+/- lakes), branching capillaries, mineralization, and nuclear molding. To be classified as an astrocytoma >80% of the tumor must meet the criteria of one or more of the following criteria: Oval to elongate nuclei (angular) with an open faced chromatin pattern, pleomorphic cells (large nucleoli, multinucleated cells), eosinophilic and abundant cytoplasm (gemistocytic), elongate cells (pilocytic), naked nuclei, random and disorganized patterns, spindle cell morphology, rare mucin microcysts (well-defined), mineralization, and eosinophilic stroma (fibrillary). Gliomas exhibiting a biphenotypic/biphasic morphology with both phenotypes in high proportions (>30-40% each) or an undifferentiated cellular morphology are classified as an "undefined glioma".6
Once the glial tumor type is determined, the neoplasm's infiltration into the adjacent parenchyma is assessed at low magnification and classified as either "none, focal, or diffuse".6
The neoplasm is then graded as either "high" or "low" based on multiple criteria. All gliomas with necrosis (excluding single cell) and/or microvascular proliferation +/- pseudopalisading are classified as high grade. Pseudopalisading necrosis occurs when neoplastic cells line up perpendicularly to areas of geographic necrosis. Microvascular proliferation may be characterized as convoluted and glomeruloid vasculature, multilayered and hypertrophied endothelial cells, and vascular arcades. Microvascular proliferation must be distinguished from reactive vasculature, with multiple layers of cells and high degree of tortuosity being critical distinguishing features. Neoplasms lacking these features are further assessed for any mitoses in ten 400x fields and/or overt features of malignancy (e.g. nuclear pleomorphism, anisokaryosis, anisocytosis, and cellular atypia). The presence of any of these features are consistent with a high grade glioma whereas their absence is consistent with a low grade glioma.6
References:
1. Brat DJ, Perry A. Astrocytic and Oligodendroglial Tumors. In: Practical Surgical Neuropathology: A Diagnostic Approach. Elsevier; 2018:91?123.
2. Cantile C, Youssef S. Nervous System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, Vol. 1. 6th ed. St. Louis, MO: Elsevier, 2016: 250-406.
3. Hicks J, Platt S, Kent M, et al. Canine brain tumours: a model for the human disease?: Canine brain tumours. Veterinary and Comparative Oncology 2017;15:252?272.
4. Higgins RJ, Bollen AW, Dickinson PJ, Siso-Llonch S. Tumors of the Nervous System. In: Meuten DJ, ed. Tumors of Domestic Animals. 5th ed. Ames, IA: Wiley; 2017: 834-891.
5. Johnson GC, Coates JR, Wininger F. Diagnostic Immunohistochemistry of Canine and Feline Intracalvarial Tumors in the Age of Brain Biopsies. Veterinary Pathology 2014;51:146?160.
6. Koehler JW, Miller AD, Miller CR, et al. A Revised Diagnostic Classification of Canine Glioma: Towards Validation of the Canine Glioma Patient as a Naturally Occurring Preclinical Model for Human Glioma. J Neuropathol Exp Neurol. 2018;77(11):1039-1054.
7. Lipsitz D, Higgins RJ, Kortz GD, et al. Glioblastoma Multiforme: Clinical Findings, Magnetic Resonance Imaging, and Pathology in Five Dogs. Veterinary Pathology 2003;40:659?669.
8. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathologica 2016;131:803?820.
9. Perry A, Rosenblum MK. Central Nervous System. In: Rosai and Ackerman's Surgical Pathology.11th ed. Philadelphia, PA: Elsevier; 2018: 1948-2085.
10. Ramos-Vara JA, Borst LB. Immunohistochemistry: Fundamentals and Applications in Oncology. In: Meuten DJ, ed. Tumors of Domestic Animals. 5th ed. Ames, IA: Wiley; 2017: 834-891.
11. Reitman ZJ, Olby NJ, Mariani CL, et al. IDH1 and IDH2 hotspot mutations are not found in canine glioma. International Journal of Cancer 2010;127:245?246.
12. R?thlisberger A, Lehmbecker A, Beineke A, et al. Suspected primary glioblastoma multiforme in the canine spinal cord. Journal of Small Animal Practice 2012;53:604?607.
13. Snyder JM, Shofer FS, Winkle TJ, et al. Canine Intracranial Primary Neoplasia: 173 Cases (1986-2003). Journal of Veterinary Internal Medicine 2006;20:669?675.
14. Song RB, Vite CH, Bradley CW, et al. Postmortem Evaluation of 435 Cases of Intracranial Neoplasia in Dogs and Relationship of Neoplasm with Breed, Age, and Body Weight. Journal of Veterinary Internal Medicine 2013;27:1143?1152.
15. Stoica G, Levine J, Wolff J, et al. Canine Astrocytic Tumors: A Comparative Review. Veterinary Pathology 2011;48:266?275.
16. Vandevelde M, Higgins RJ, Oevermann A. Veterinary Neuropathology: Essentials of Theory and Practice. Ames, IA: Wiley-Blackwell; 2012.