Signalment:
Gross Description:
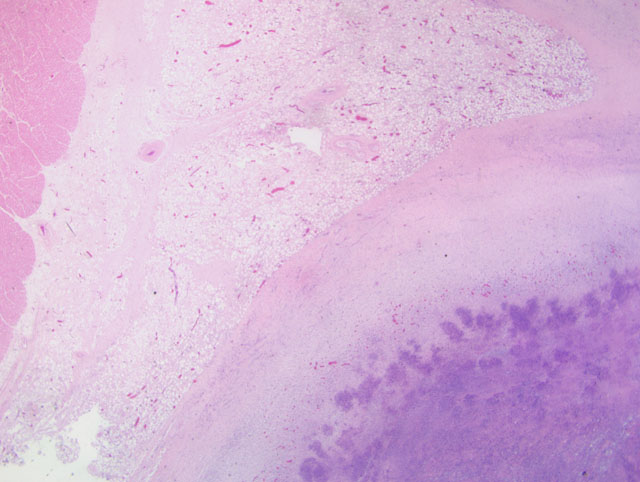
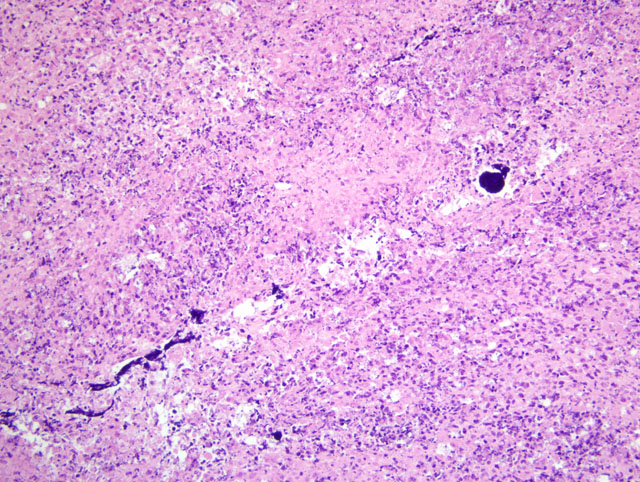
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
C. pseudotuberculosis often enters the body through skin or oral wounds. The draining lymph node is then infected and subsequently the organism can spread to internal organs, typically the lungs. Treatment of animals with C. pseudotuberculosis often results in limited success due to the intracellular nature of the organism and the formation of abscesses leading to poor penetration of the drug. Typically, once the bacterium enters the lymph nodes, the infection is considered persistent except for the rare occasion when the node ruptures and drains through the skin to the outside of the body. A primary finding of pericarditis without lymphadenitis, as in this case, is unusual.
JPC Diagnosis:
Conference Comment:
Conference participants briefly reviewed bacteria able to survive in macrophages, including Rhodococcus spp., Salmonella spp., Mycobacteria spp., Brucella spp. and Listeria spp. Other less commonly encountered microbial agents in macrophages include rickettsial organisms, Shigella spp., Trypanosomaspp., Coxiella spp., chlamydial organisms, Toxoplasma spp., and Legionella spp.
The contributor provides a succinct synopsis of the pathogenesis of CLA in sheep; readers are referred to a comprehensive review by Baird and Fontaine(1) for more details. In addition to phospholipase D, C. pseudotuberculosis produces a waxy mycolic acid coat with well-described cytotoxic properties, which both contributes substantially to the organisms pathogenicity and enhances its ability to survive for extended periods in the environment. This latter attribute is common to the actinomycete family, of which the genera Corynebacterium, Mycobacterium, Rhodococcus, and Nocardia are members.(1)
In sheep, C. pseudotuberculosis has been hailed the perfect parasite because of its ability to evade the immune system, establish a chronic infection that often persists for the life of the host, and readily infect flock mates, all while only rarely producing fatal infections. Therefore, in regions where the disease is endemic, CLA may be of considerable economic importance. Visceral lesions are far less common than superficial lymphadenitis in both sheep and goats, and they occur even less frequently in goats as compared to sheep. While abscesses in sheep due to CLA are most often found in the superficial lymph nodes of the torso, in goats the lesions manifest as lymphadenitis of the superficial nodes of the head and neck.(1)
In horses, C. pseudotuberculosis causes three distinct disease syndromes: 1) ulcerative lymphangitis; 2) contagious folliculitis and furunculosis, which is also known as equine contagious acne, Canadian horsepox, and equine contagious pustular dermatitis; and 3) deep-seated subcutaneous abscessation, also termed pigeon fever, Wyoming strangles, and false strangles. In farmed llamas and alpacas in South America, C. pseudotuberculosis is a commonly identified cause of purulent lymphadenopathy. In dairy cattle in Isreal, C. pseudotuberculosis has been implicated in several syndromes, including deep subcutaneous abscesses and mastitis. Sporadic and experimental infections have been reported in a variety of species.(1)
References:
2. DAfonseca VD, Moraes PM, Corella FA, Pacheco LG, Meyer R, Portela RW, Miyoshi A, Azevedo V: A description of genes of Corynebacterium psuedotuberculosis useful in diagnostics and vaccine applications. Gen and Mol Res, 7:252-260, 2008
3. Valli VEO: Hematopoietic system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 3, pp. 292-294. Elsevier Saunders, Philadelphia, PA, 2007