CASE 2: H19-107A (JPC 4141185-00)
Signalment:
A 4-year-old female entire Bengal cat
History:
The cat was submitted to the anatomic pathology diagnostic service after sudden unexpected death.
Gross Pathology:
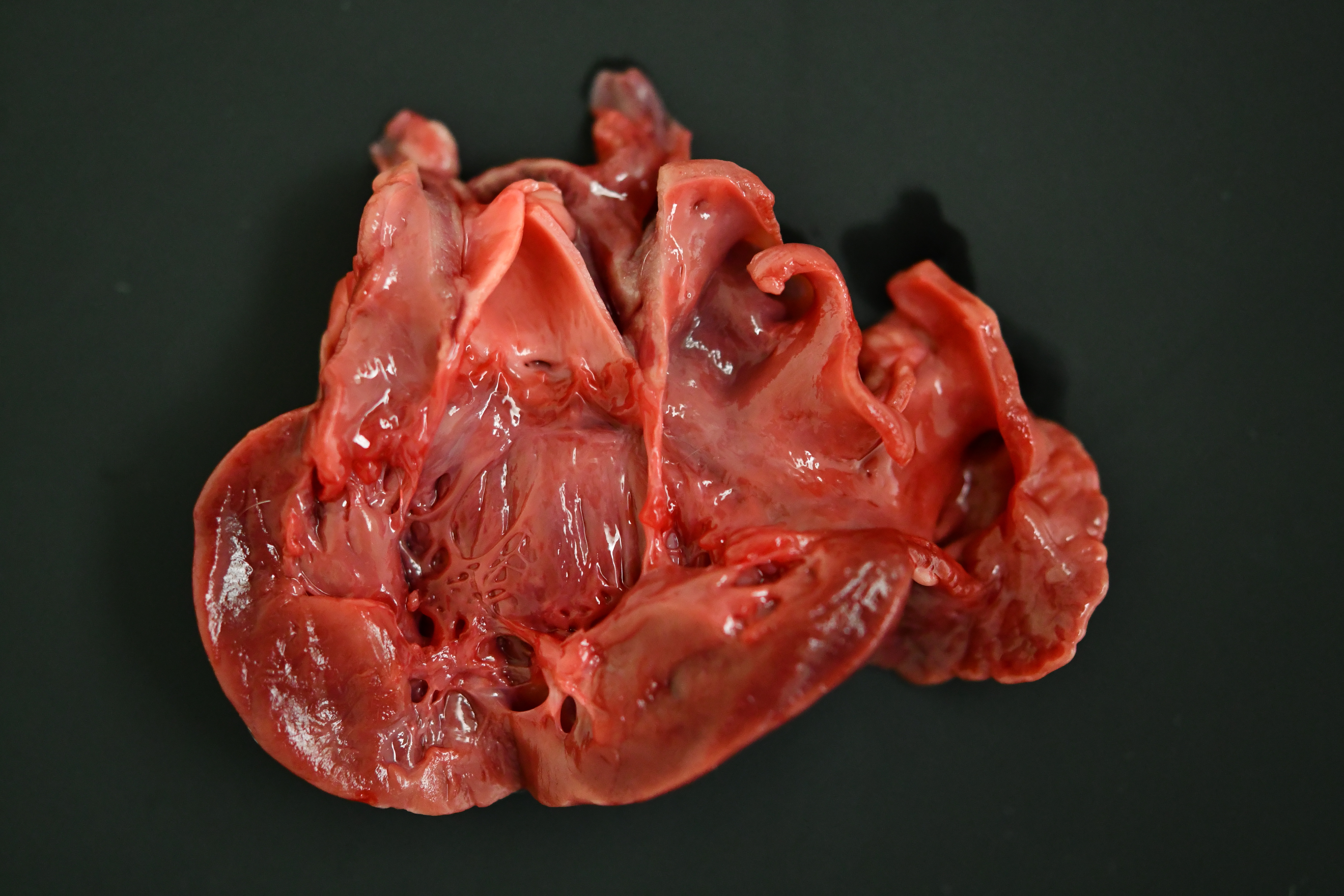
The cat was in heavy body condition (4/5) and there was minimal post-mortem decomposition. Multifocal, haphazardly arranged, approximately 1-3 mm diameter thick, firm to hard bands of smooth tissue were present running between the endocardial surfaces of the left ventricle near the apex (endomyocardial fibrosis). The total weight of the heart was 21.9 g (0.49% of total bodyweight, within normal limits). The left auricle was approximately 1.5 times the diameter of the right auricle (left auricular dilation). There was a focal, pale, irregularly shaped focus of myocardium measuring approximately 0.5 cm x 0.5 cm x 0.5 cm within the apex of the left ventricle (myocardial necrosis, presumptive). Approximately 1 ml of dark, red-tinged fluid was present within the pericardial sac (post-mortem fluid accumulation vs. pericardial effusion). Approximately 15 ml of red-tinged fluid was present within the thoracic cavity (hydrothorax). The lungs were reduced in size, occupying approximately 60-70% of the thoracic cavity (atelectasis). The lungs were diffusely mottled, dark red to tan (post-mortem autolysis vs. atelectasis). A small amount of frothy material was found within the entire length of the trachea, and the pulmonary parenchyma oozed a small amount of clear, dark red tinged fluid on transection (pulmonary edema).
Laboratory results:
None performed.
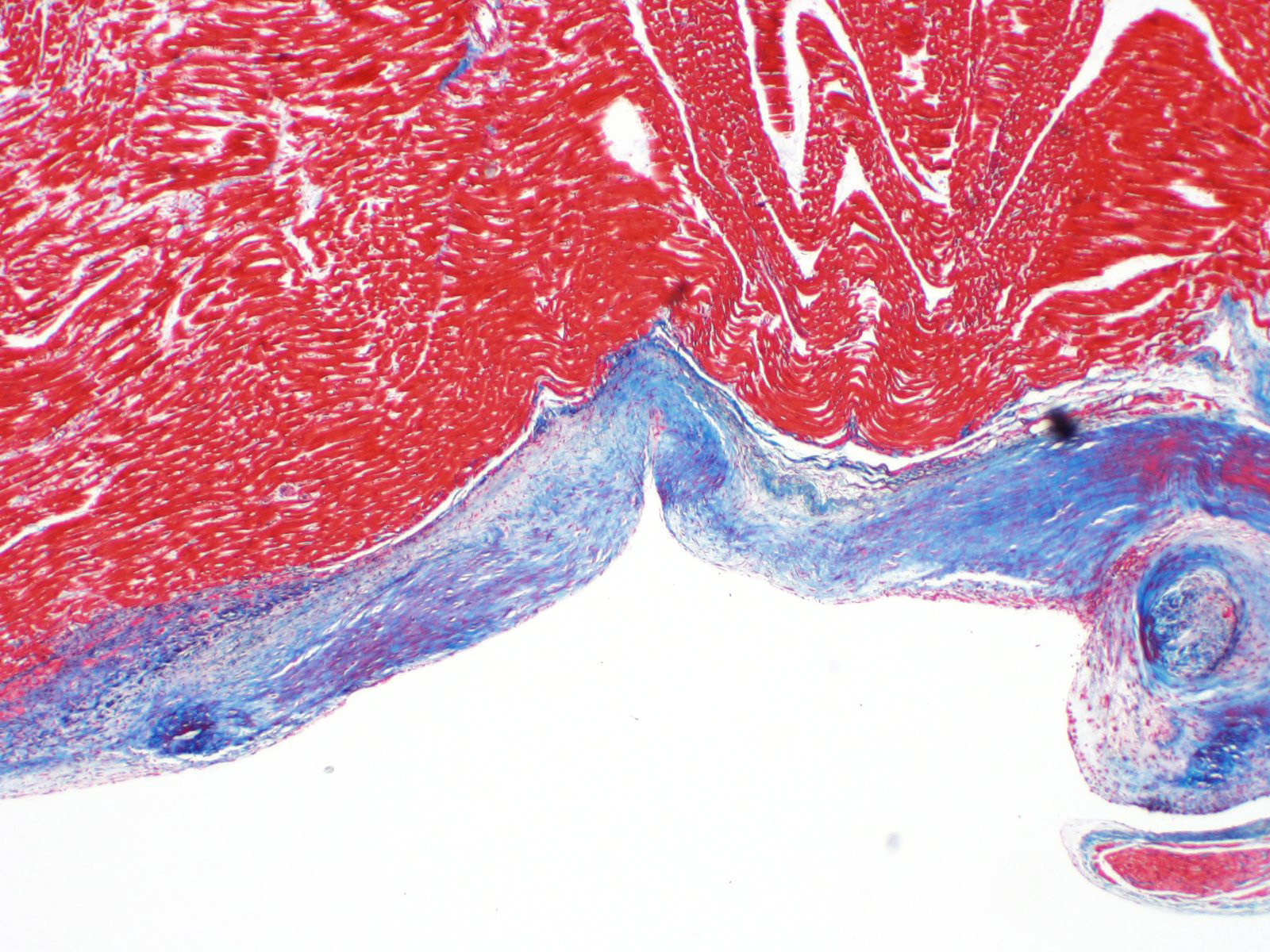
Microscopic description:
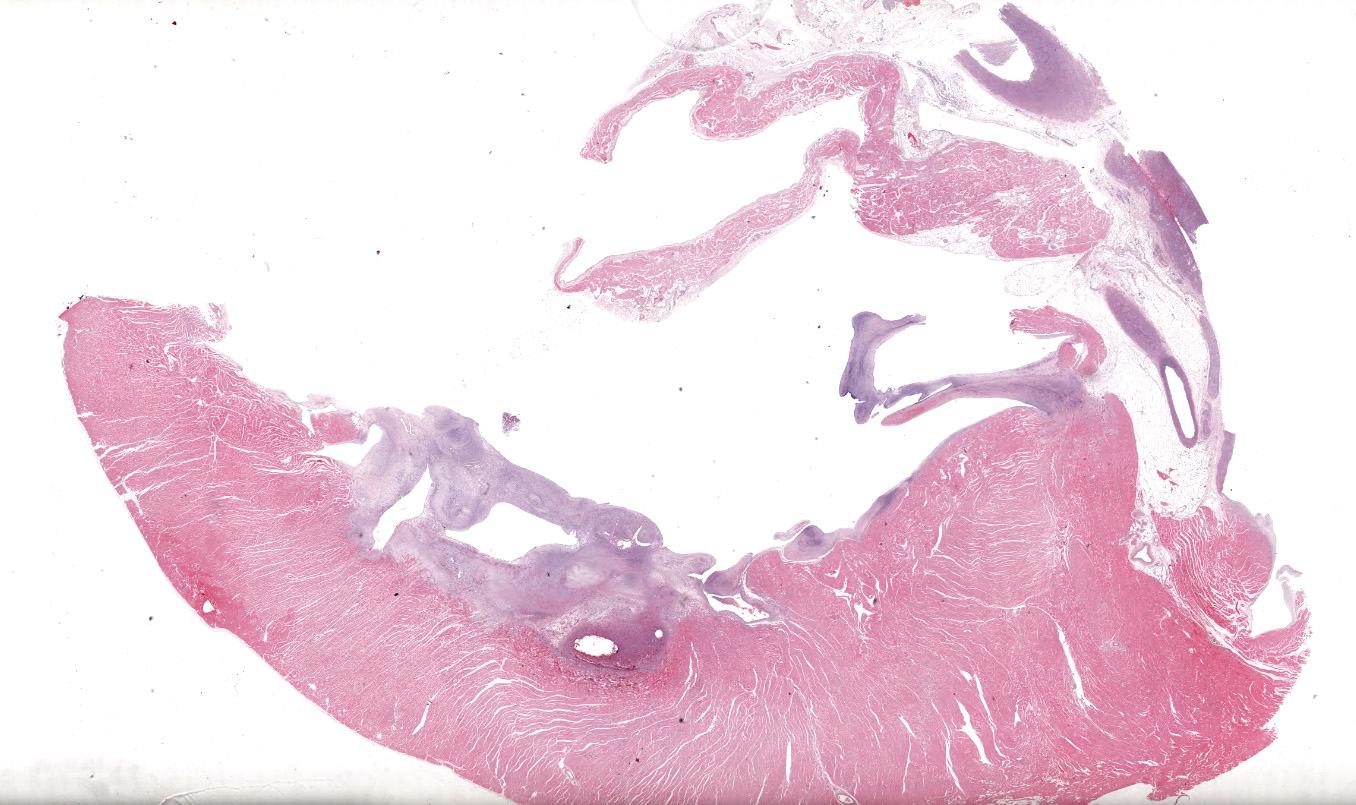
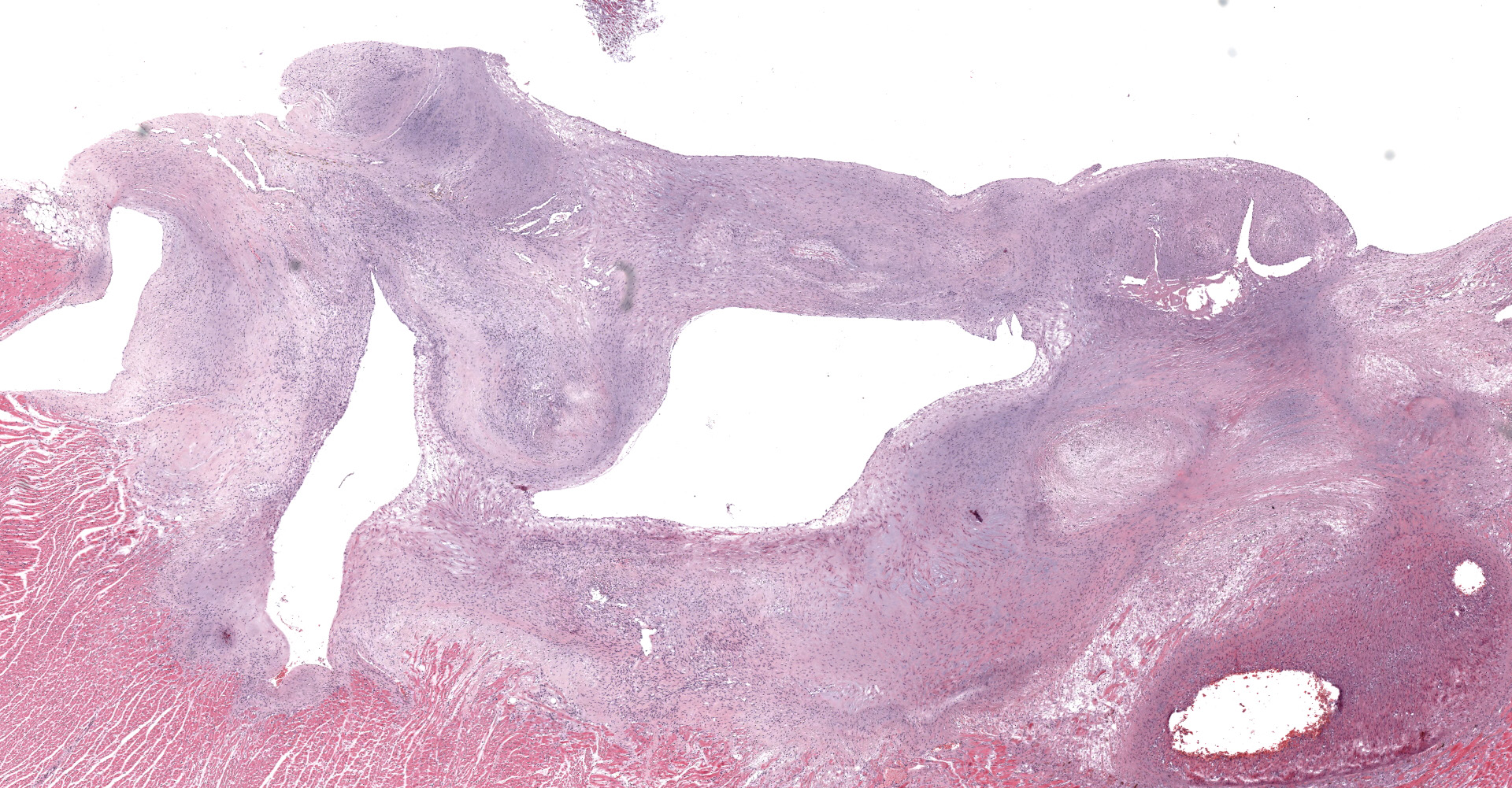
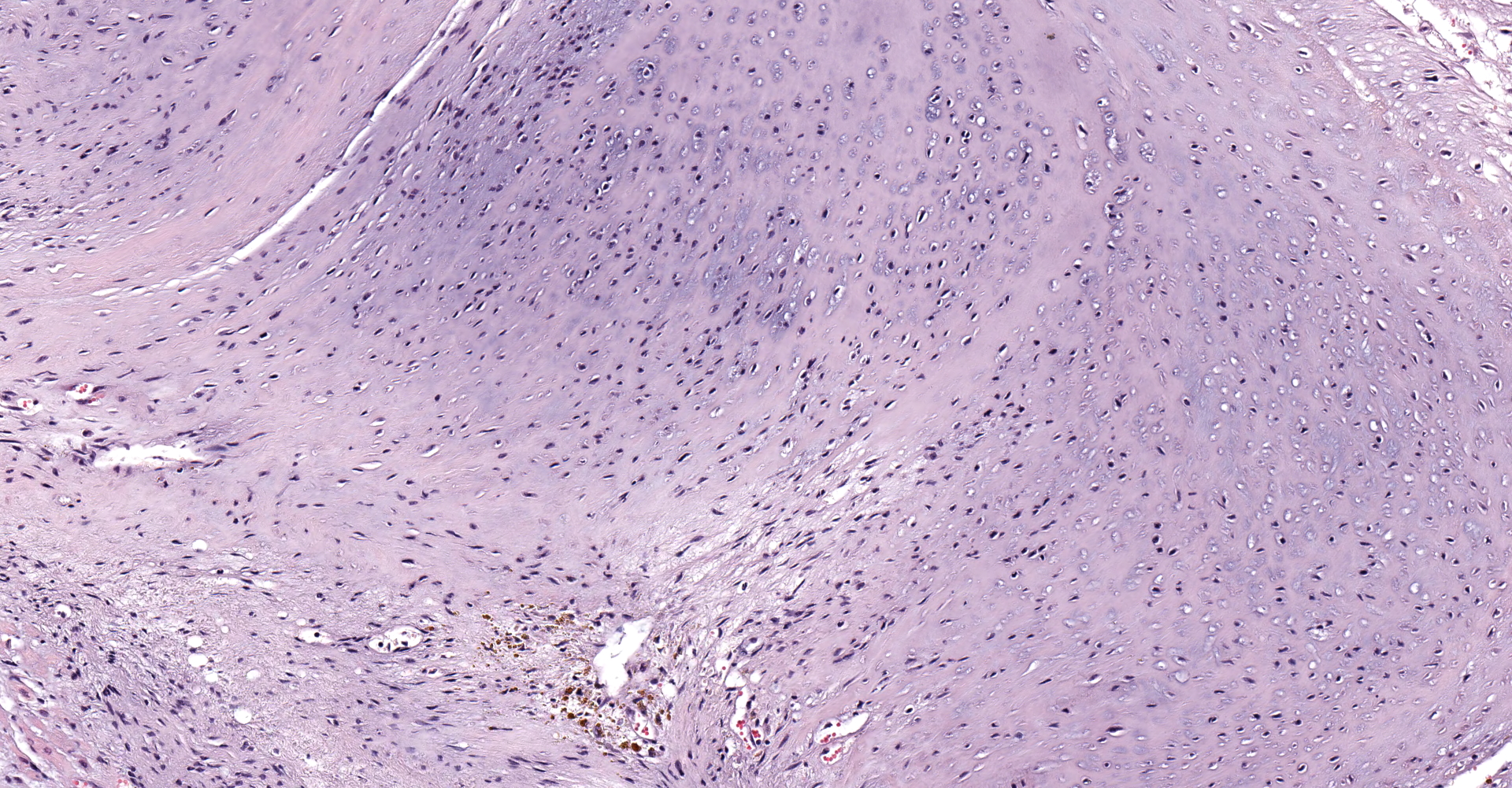
A single section of the left ventricle and atrium are examined. Diffusely the endocardium of the left ventricle and mitral valve is moderately to markedly expanded by moderate amounts of variably dense fibrous connective tissue with multifocal mineralization and chondroid differentiation. This fibrous connective tissue also multifocally infiltrates into the underlying myocardium, occasionally surrounding and isolating individual and small groups of cardiac myocytes. Multifocally scattered throughout the expanded endocardium there are variably sized roughly circular occasionally cavitated formations of swirling spindle cells with associated collagen (recanalized blood vessels vs. atypical endocardial formations). Small amounts of pale eosinophilic fibrillar material, which stains bright red on M.S.B. histochemistry (fibrin) is present either within the walls or lumens of these structures (thrombosis). Low numbers of lymphocytes, plasma cells, histiocytes and occasional neutrophils are multifocally scattered throughout the epicardial adipose.
Contributor's morphologic diagnosis:
Heart: Severe, multifocal, chronic, left ventricular and mitral valvular endocardial to subendocardial fibrosis with chondroid metaplasia and multifocal thrombosis
Contributor's comment:
Histopathology is consistent with a diagnosis of left ventricular and mitral valvular fibrosis, also known as restrictive cardiomyopathy. Secondary changes including left atrial dilation, pulmonary edema, hydropericardium and hydrothorax indicate that the patient was in congestive heart failure at the time of death. There was no gross evidence of aortic or iliac bifurcation thromboembolism in this case.
Following hypertrophic cardiomyopathy, restrictive cardiomyopathy is the second most common form of cardiomyopathy in cats.3 Restrictive cardiomyopathy refers to any pathologic process which leads to restricted filling and reduced diastolic volume of one or both ventricles.10 Affected animals will often have normal left ventricular wall thickness and normal or minimally decreased systolic function.7 Similar to humans, restrictive cardiomyopathy can be categorized either as a myocardial form (in which myocardial involvement predominates) or the more common endomyocardial form (in which endocardial involvement predominates).6 The most common pathologic processes causing restrictive cardiomyopathy in cats include endomyocardial scar formation and myocardial fibrosis, however infiltrative processes have also been reported.5
In left ventricular endomyocardial restrictive cardiomyopathy, the fibrotic scar tissue typically involves the left ventricular apex and outflow tract including both the left ventricular free wall and interventricular septum.8 Diffuse endomyocardial fibrosis, cardiomyocyte degeneration, infarction, and inflammation are less commonly described.8 Biventricular involvement is rare in cases of endomyocardial restrictive cardiomyopathy.
The etiopathogenesis of feline restrictive cardiomyopathy remains unknown. A continuum between endomyocarditis and endomyocardial fibrosis has been proposed due to age relationships and common lesion distribution.4 Whilst canine parvovirus has been associated with myocarditis leading to myocardial fibrosis in dogs, in recent publication McEndaffer et al (2017) demonstrated no association with feline panleukopenia virus and restrictive cardiomyopathy in cats.8 The endomyocardial form of restrictive cardiomyopathy in cats has been described in numerous breeds, however specific breed predispositions have not been reported.6
Prognosis for cats with endomyocardial restrictive cardiomyopathy is poor with many affected cats dying from either congestive heart failure or aortic thromboembolism.6 A retrospective study of feline endomyocarditis and left ventricular endocardial fibrosis in one university pathology service reported these pathologies as the cause of death in >4% of cats compared to 2.3% of cats having died from hypertrophic cardiomyopathy and 2.2% of cats within the service having died from dilated cardiomyopathy.12
Contributing Institution:
Department of Veterinary Anatomic Pathology
The Animal Hospital at Murdoch University
https://theanimalhospital.com.au/pathology-services
JPC diagnosis:
Heart, left ventricle, endocardium: Fibrosis, diffuse, severe with chondroid metaplasia.
JPC comment:
Feline restrictive cardiomyopathy (RCM), like hypertrophic cardiomyopathy (HCM), is a disease of diastolic dysfunction. In human medicine, the endomyocardial form of RCM is subclassified into two forms, Loeffler endomyocarditis, and endomyocardial fibrosis. Both have similar and histologically indistinguishable endpoints, but different pathogeneses. Interestingly, there is also a geographic distinction between these two processes, with the Loeffler endomyocarditis most common in temperate climates, and endomyocardial fibrosis most common in equatorial Africa. In Loeffler endomyocarditis, fibrosis is the result following release of granules from infiltrating eosinophils in early stages of the disease, and is associated with hypereosinophilia, thromboembolism, and arteritis. Endomyocardial fibrosis usually affects younger patients and is not associated with eosinophilia.11
Research in human restrictive cardiomyopathy has suggested a number of different genetic mutations that can lead to disease. One revealed mutation (p.Y122H) occurs in the DES gene, a highly conserved coil-1 subdomain of desmin, and results in defective filament production by myocytes.1 Other implicated mutations occur in the MYH7, ABCC9,9 and MYL2.13 Therapies addressing these mutations are in active development. The relevance to feline RCM remains to be determined.
RCM may be secondary to certain infiltrative conditions, such as amyloidosis or sarcoidosis. However, in the race to address SARS-CoV-2 with medical treatments, hydroxychloroquine and chloroquine were suggested and touted as effective treatments. These two drugs were originally developed as anti-malarial medications but are now used for the treatment of rheumatoid arthritis and systemic lupus erythematosus. One noted adverse effect from these medications includes cardiotoxicity, and restrictive cardiomyopathy, dilated cardiomyopathy, and conduction abnormalities have been recorded. In these cases, presenting clinical signs may be non-specific, so endomyocardial biopsy is the key diagnostic step to perform.2
The conference moderator shared an immunohistochemical stain of the tissue, highlighting most of the proliferative tissue as smooth muscle actin positive. Conference participants and the moderator, in conjunction with an outside consultation, suggest the cells may be reparative cardiac myofibroblasts, and are hyperplastic in the face of a chronic stimulus. Conditions considered potentially likely included hypertension, or turbulence due to remodeling. Alternatively, this cat may have had a genetic connective tissue anomaly that contributed to this lesion.
References:
- Brodehl A, Hakimi SAP, Stanasiuk C, et al. Restrictive cardiomyopathy is caused by a novel homozygous desmin (DES) mutation p.Y122H leading to a severe filament assembly defect. Genes. 2019;10:918.
- Dogar MU, Shah NN, Ishtiaq S, et al. Hydroxychloroquine-induced restrictive cardiomyopathy: a case report. Postgrad Med J. 2018;94(1109):185-186.
- Ferasin L, Sturgess CP, Cannon MJ, et al. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994-2001). Journal of feline medicine and surgery; 2003, 5: 151?159.
- Fox PR, Basso C, Thiene G, et al. Spontaneously occurring restrictive nonhypertrophied cardiomyopathy in domestic cats: a new animal model of human disease. Cardiovascular Pathology; 2014, 23(1), 28?34.
- Gallo P, d'Amati G, Cardiomyopathies. In: Cardiovascular Pathology, 3rd Edit., MD Silver, AI Gotlieb, FI Shoen, Eds., Churchill Livingstone, Elsevier, New York; 2001, 285-325.
- Kimura Y, Fukushima R, Hirakawa A, et al. Epidemiological and clinical features of the endomyocardial form of restrictive cardiomyopathy in cats: a review of 41 cases. Journal of Veterinary Medical Science. 2016, 15, 373.
- Locatelli C, Pradelli D, Campo G, et al. Survival and prognostic factors in cats with restrictive cardiomyopathy: a review of 90 cases. Journal of feline medicine and surgery; 2018, 20(12),1138-43.
- McEndaffer L, Molesan A, Erb H, et al. Feline panleukopenia virus is not associated with myocarditis or endomyocardial restrictive cardiomyopathy in cats. Veterinary pathology; 2017, 54(4),669-675.
- Neagoe O, CiobanuA, Diaconu R, Mirea O, Donoiu I, Militaru C. A rare case of familial restrictive cardiomyopathy with mutations in MYH7 and ABCC9 genes. Discoveries. 2019;7(3):e99.
- Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation; 1996, 93, 841-842.
- Robinson WF, Robinson NA. Cardiovascular System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, Vol 3, 6th Ed. St. Louis, MO:Elsevier. 2016:45.
- Stalis IH, Bossbaly MJ, Van Winkle TJ. Feline endomyocarditis and left ventricular endocardial fibrosis. Veterinary Pathology; 1995, 32(2), 122?126.
- Zaleta-Rivera K, Dainis A, Ribeiro AJS, et al. Allele-specific silencing ameliorates restrictive cardiomyopathy due to a human myosin regulatory light chain mutation. Circulation. 2019;140(9):765-778.