Signalment:
8-year-old male rhesus monkey (
Macaca mulatta)This SIV-infected monkey had a several day history of reduced cage movement and lower body stiffness. The animal was humanely sacrificed and submitted for necropsy evaluation.
Gross Description:
The bladder was markedly distended at necropsy. Small areas of superficial congestion were noted from the meningeal surface in the distal lumbar cord and cauda equina region. On transection of fixed cord for trimming, more extensive punctate and focally extensive areas of hemorrhage were seen within the spinal nerves, especially in the more inferior cauda equina areas.
Histopathologic Description:
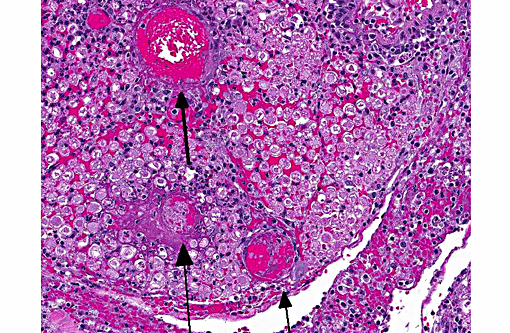
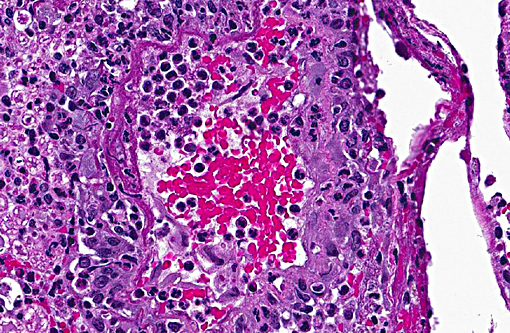
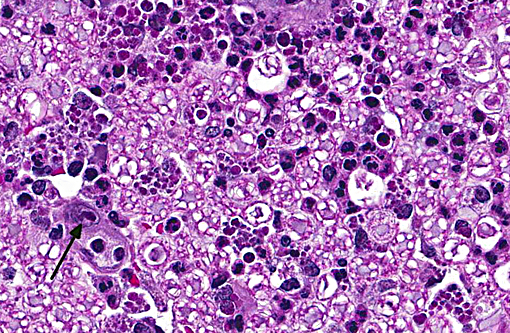
Sections distributed and corresponding lesions vary somewhat as to the cord level present, with some slides containing only cauda equina tissue without distal lumbosacral spinal cord body. A patchy to focally extensive acute, necrotizing and inflammatory process is noted. Abundant neutrophilic inflammation is present within spinal nerves, admixed with macrophages and globular eosinophilic debris. Within some nerve roots (and some sections of cord), occasional swollen and/or fragmented axons are present. Numerous vessels are infiltrated by dense populations of neutrophils and fibrinoid necrosis and debris are frequently seen within and surrounding vascular walls. Associated with this angiitis are infrequent thrombi. Scattered throughout the section are large, eosinophilic to amphophilic intranuclear inclusion bodies within enlarged (cytomegalic) Schwann cells and infrequently in endothelium. Also noted in most slides is patchy, necrotizing and fibrinous meningitis. Some sections contain mixed macrophage and granulocytic inflammatory debris within the distal aspect of the central canal.
Morphologic Diagnosis:
1) Radiculitis, necrotizing and fibrinous, acute, patchy to focally extensive, marked to severe with cytomegaly and intranuclear inclusion bodies, eosinophilic and amphophilic, (Cowdry type A)
2) Angiitis, necrotizing, neutrophil-rich, acute, multifocal with areas of fibrinoid change within and surrounding vessel walls and foci of thrombosis
3) Meningitis, necrotizing and fibrinous, patchy, mild-moderate (most sections)
4) Mixed inflammatory infiltrates, mild-moderate, central canal (some sections)
5) Nerve fiber degeneration, multifocal, mild (some sections)
Condition:
Cytomegalovirus, rhesus macaque
Contributor Comment:
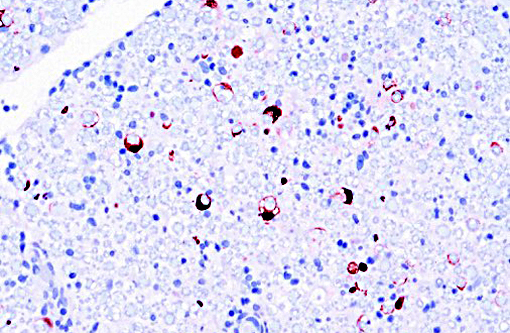
Immunohistochemical evaluation of sections was strongly positive for rhesus monkey cytomegalovirus (RhCMV) antigen. Rhesus monkey populations are infected with the betaherpesvirus RhCMV at an incidence approaching 100%, with seroconversion generally occurring within the first year of life.(5) Viral shedding in urine and saliva continues throughout life.(2) Once infected, normal host immune response is generally able to control the virus, although not eradicate it.(5) Immunosuppression such as that experienced with SIV infection leads to latent CMV reactivation. The developing rhesus macaque brain is also susceptible to RhCMV infection in the second trimester and intrauterine exposure result in neuropathic outcomes similar to those observed in human congenital CMV infection.(7)
Virus presence has been documented within the gastrointestinal tract, spleen, lung, central nervous system, liver, lymph nodes, testicles, spleen, intestine, nerves and arteries.(4) It is the most frequently identified viral opportunistic pathogen in rhesus macaques.(3) Either multifocal or diffuse interstitial pneumonitis is the most commonly detected lesion seen.(2) Unfortunately in this animal, other tissues were not submitted to determine the extent of organs involved. Infection may also lead to the formation of discrete proliferative masses in the gastrointestinal tract.(3)
From a comparative perspective, human CMV accounts for most HIV-related radiculitis, which in AIDS patients, tends to involve lumbosacral nerve roots, producing a rapidly progressive cauda equine syndrome with severe lower back pain.(8) In humans, highly active antiretroviral therapy (HAART), first introduced in the mid 1990s has dramatically reduced the risk of opportunistic infections and improved the prognosis of patients with HIV infection. CMV disease has become a rare complication in AIDS patients.(6)
JPC Diagnosis:
Lumbar spinal roots: Radiculitis, necrotizing and neutrophilic, multifocal to coalescing, with necrotizing vasculitis, fibrinohemorrhagic meningitis, and karyomegalic intranuclear viral inclusions.
Conference Comment:
The most common opportunistic viral infection in SIV-infected rhesus macaques is rhesus cytomegalovirus (RhCMV). While it has been well documented to cause lesions in many organs including the leptomeninges and spinal nerve roots, as seen in this case, its role in peripheral neuropathies is less well researched. A recent study of RhCMV associated facial neuritis noted a mixed inflammatory population composed of neutrophils and macrophages, with macrophages containing intranuclear inclusion bodies.(1) Lesions were present in nerves of the tongue, lacrimal gland and other facial tissues. Axon loss was proportional to the degree of inflammation, the neuritis associated macrophages were infected with RhCMV and there was absence of evidence to support infection of Schwann cells.(1) Lesions were consistent with demyelination and loss of axons secondary to inflammation, supporting the studys assertion that nerve damage is likely related to inflammation rather than direct viral infection of Schwann cells in RhCMV peripheral neuropathies.(1) Lesions in those cases varied in severity from effacement of nerve architecture to milder lesions localized to the periphery. As mentioned above, human CMV (HCMV) is also associated with radiculoneuritis and lesions include peripheral neuritis, with the facial nerves being most commonly affected; however, in HCMV associated neuritis there is a demonstrated viral predilection for Schwann cells.(1)
Conference participants noted that the spinal cord was largely unaffected and they described the inflammatory infiltrate and hemorrhage as extending from affected spinal nerves into adjacent perineural connective tissue and meninges. Additionally, the inflammatory infiltrate was identified as primarily neutrophilic. This is attributed to RhCMV induction of a CXC chemokine, which results in neutrophil chemoattraction.(1) Other microscopic features noted by participants included dilated myelin sheaths with numerous spheroids, variable numbers of gitter cells, and low numbers of cyto- and karyomegalic cells (most likely Schwann cells in this case), with large, darkly eosinophilic, intranuclear viral inclusion bodies.. Other commonly affected tissues include the lung, gastrointestinal tract and the testes.
References:
1. Assaf BT, Knight HL, Miller AD. Rhesus cytomegalovirus (Macacine herpesvirus-3) associated facial neuritis in a simian immunodeficiency virus infected
rhesus macaques. Vet Pathol. 2015; 52(1):217-223.
2. Baskin G. Disseminated cytomegalovirus infection in immunodeficient rhesus monkeys. Am J Pathol. 1987; 129(2):345-352.
3. Hendricks Hutto E, Anderson D, Mansfield K. Cytomegalovirus associated discrete gastrointestinal masses in macaques infected with simian immunodeficiency virus. Vet Pathol. 2004; 41:691-695.
4. Kuhn E, Stolte N, Matz-Rensing K, Stahl-Hennig C. et al. Immunohistochemical studies of productive rhesus cytomegalovirus infection in rhesus monkeys (Macca mulatta) infected with simian immunodeficiency virus. Vet Pathol. 1999; 36:51-56.
5. Powers C, Frick K. Rhesus CMV: An emerging animal model for human CMV. Med Microbiol Immunol. 2008; 197:109-115.
6. Salzberger B, Hartmann P, Hanies F, Uyanik B et al. Incidence and prognosis of CMV disease in HIV-infected patients before and after introduction of combination antiretroviral therapy. Infection. 2005; 33(5/6):345-349.
7. Tarantal A, Salmat M, Britt W, Lucaw P, et al. Neuropathogenesis induced by rhesus cytomegalovirus in fetal rhesus monkeys (Macca mulatta). J Infect Dis. 1998; 177:446-450.
8. Tarulli A, Raynor E. Lumbosacral radiculopathy. Neurol Clin. 2007:25:387-405.