WEDNESDAY SLIDE CONFERENCE 2022-2023
Conference #15
Case 1:
Signalment:
3-month-old, male, Lewis rat (Rattus norvegicus)
History:
This rat was part of a study receiving high-dose whole body irradiation daily for six days. Five days after the last exposure, the animal presented quiet, with thin body condition (BSC 1/5), a hunched appearance, and an unkempt haircoat. Due to lack of improvement with supportive care, the animal was euthanized two days later and submitted for necropsy evaluation.
Gross Pathology:
On gross examination, the cecum was slightly distended and doughy, and there was a small amount of fecal staining at the ventral tail base. There were several formed fecal pellets present in the colon. The liver was mildly and diffusely pale.
Laboratory Results:
On CBC analysis, there was mild lymphopenia, mild neutrophilia, and moderate monocytosis. In addition, there was a mild decrease in HCT and MCV, and a mild increase in MCHC, representing a microcytic, hypochromic anemia.
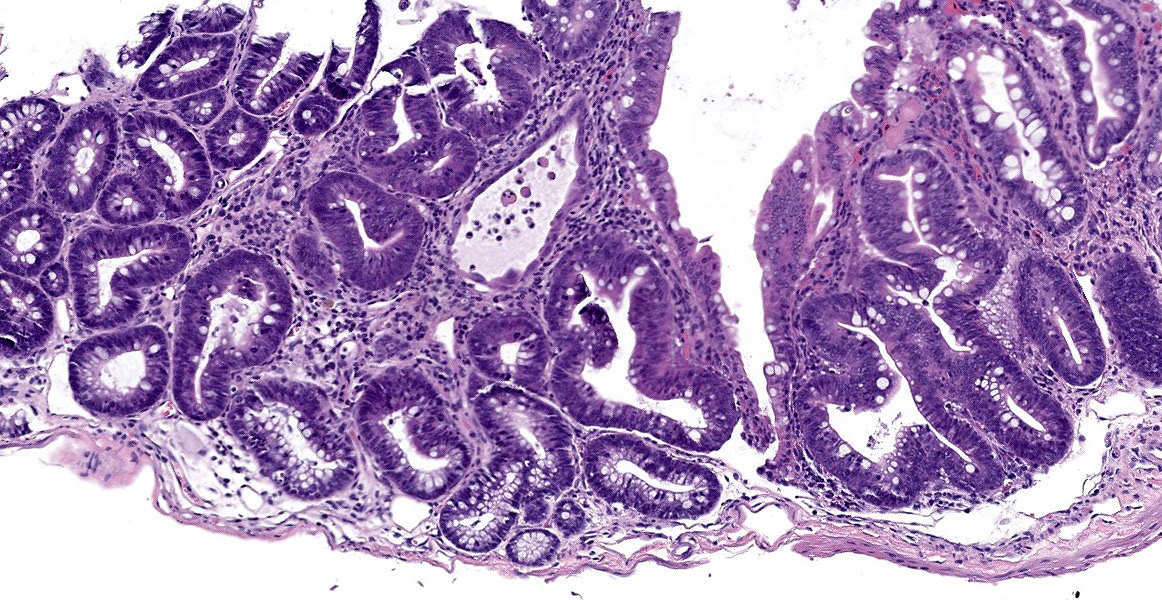
Microscopic Description:
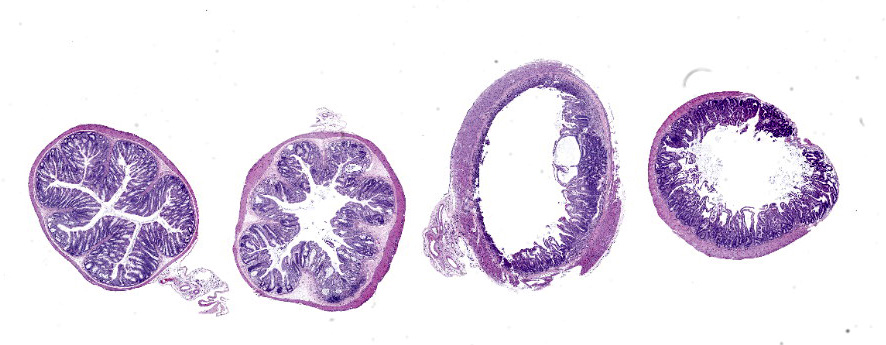
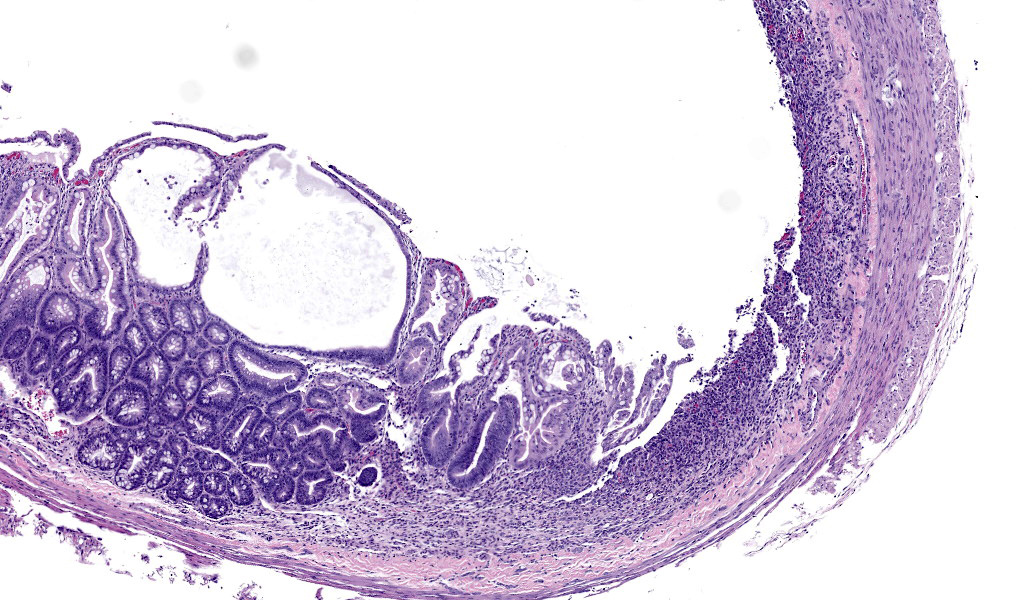
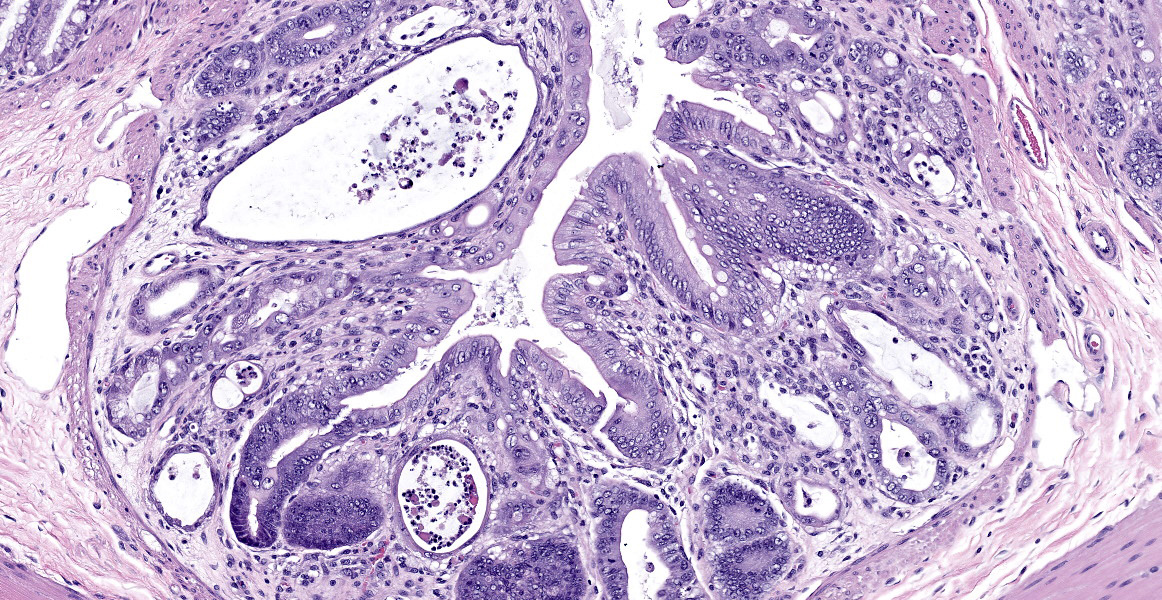
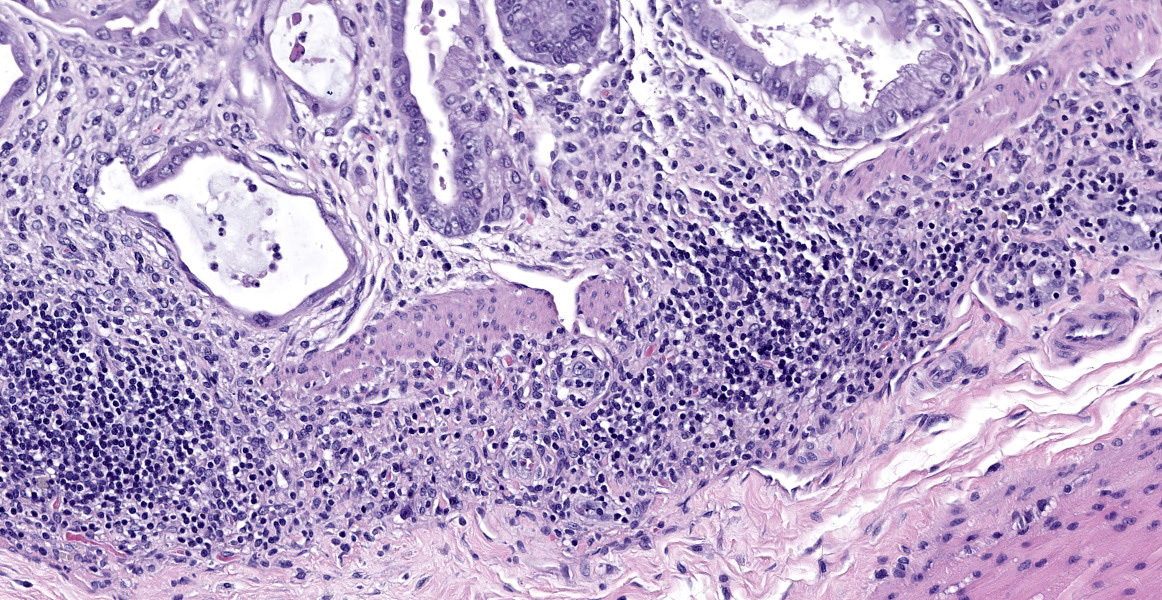
In sections of small intestine, there was loss of normal villus architecture characterized by moderate and multifocal villus blunting and fusion, with replacement of areas of lamina propria with loose collagen and granulation tissue. The deep crypt epithelium was hyperplastic with large, plump crypt epithelial cells that showed piling and disorganization with frequent mitotic figures. Within the ileum, similar changes were present within the lamina propria and crypt epithelium, with the additional finding of severe, locally extensive mucosal ulceration with replacement by granulation tissue. There was diffuse and moderate lymphangiectasia within small intestinal villi, and marked depletion of mucosal associated lymphoid tissue. Within the colon, there was marked degeneration and necrosis of the superficial mucosal and deep glandular epithelium with the presence of necrotic debris present within glandular lumens. There was moderate granulation tissue throughout the lamina propria of the colon, with attenuation, loss, and regeneration of colonic glandular epithelium. There was mild edema present within the submucosa, and severe lymphoid depletion within Peyer?s patches. In the cecum, there were sporadic areas of glandular degeneration with loss of epithelium and small amounts of granulation tissue within the lamina propria. In other organs, there was minimal and multifocal myofiber degeneration representative of chronic cardiomyopathy in the heart, sporadic clusters of alveolar histiocytes within the lung, and decreased erythroid progenitors within the bone marrow.
Contributor?s Morphologic Diagnoses:
Small and large intestine: Enterocolitis, necrotizing and ulcerative, diffuse, with regeneration.
Contributor?s Comment:
This case represents lesions which result from radiation toxicity to the intestinal tract. The lesions observed here are a continuum of glandular epithelial necrosis, regeneration, and ultimately, villar blunting, fusion, and granulation tissue proliferation as a result of cytotoxicity of rapidly dividing crypt epithelial cells. In humans, radiation-induced gastrointestinal syndrome (RIGS) occurs secondary to radiation therapy for neoplasia of the abdomen and pelvis and is a major limiting factor and source of morbidity and mortality in patients receiving this therapy.
The detrimental effects of radiation exposure were first described by Walsh (1897) two years after the discovery of X-rays, who concluded that irradiation caused inflammation of the mucosa of the intestinal tract.2,16 Mechanisms underlying this side effect of radiation are still incompletely understood, and there are still no effective preventions or treatments for this condition. Every year, over 300,000 patients receive abdominal radiation for cancer treatment, and approximately 60-80% of these patients develop some form of bowel toxicity.2,8 In the process of radiation treatment for intra-abdominal or pelvic neoplasia, healthy bowel is ultimately affected by the radiation treatment, resulting in significant morbidity and mortality.13 Therefore, it is a significant limiting factor for many patients receiving radiation therapy for abdominal or pelvic neoplasia.19,26 However, radiation therapy is still a mainstay of cancer treatment, used in approximately half of cancer patients, and is therapeutically critical in ~25% of cancer cures so a better understanding of mechanisms behind RIGS and preventative measures against it are critical.8
In acute cases of RIGS, the intestine represents an important organ at early risk, because of the nature of intestinal biology. While the pathophysiology of RIGS is still not fully understood, data suggests that it arises from a complex interaction of epithelial damage, and alterations in the immune, vascular, and enteric nervous systems, influenced by host (co-morbidities such as IBD, diabetes, vascular or collagen disorders, genetic predisposition, body mass index, tobacco smoking, genetic disorders) and therapeutic factors (dose of radiation, length of bowel involved, concurrent chemotherapy, abdominal surgery).8,13 Intestinal epithelium, particularly intestinal stem cells (ISCs) have a high rate of proliferation and thus make the bowel a sensitive target for radiation toxicity.13 In acute RIGS, this phase occurs immediately following exposure and may persist for hours to several days, and results from a direct cytotoxic effect of radiation resulting in cytotoxicity of crypt epithelial cells, including stem cells, which results in epithelial cell loss, villus blunting, fusion, and impairment of epithelial barrier function with loss of electrolytes, water, protein, mucosal ulceration, and increased permeability to luminal pathogens and antigens, resulting in mucosal inflammation and ultimately systemic effects of sepsis.2,8,13,16 Compromise to the vascular architecture leads to hemorrhage and thrombosis, which precedes ischemic damage; in addition, several animal studies have shown a direct effect on the enteric nervous system, resulting in reduced transit time and intestinal ileus.13
As lesions progress, acute RIGS may resolve following cessation of radiation exposure, or in some cases (often months to several years following exposure) may become a chronic condition, secondary to progressive occlusive vasculitis, extracellular matrix remodeling, and collagen deposition resulting in atrophy of the intestinal mucosa, fibrosis, structure formation, fistula development, and intestinal obstruction or perforation; the chronic form of RIGS occurs in up to ~17% of individuals treated with abdominal/pelvic irradiation.8,13,16 In fact, a latency period of several decades (20-30 years) between radiation therapy and chronic RIGS is not an uncommon clinical phenomenon.8
While the pathophysiology of RIGS is poorly understood, a number of studies have shown a relationship between growth factor pathways, cell cycle proteins, DNA damage mediators, and developmental or stem cell pathways in the progression of disease. For example, radiation induced damage to the intestinal tract is associated with upregulation of the JNK-MAPK cell growth and proliferation pathways and decreased expression of stress-activated p38 MAPK pathways which are important for regeneration and repair of intestinal mucosal defects.26 Inhibition of the cell cycle, either through cyclin/cdk complex inhibition, or modulation of cell cycle regulators and DNA damage checkpoint mediators, appears to protect against or improve response to GI toxicity; CDK4/6 inhibitors improve survival in irradiated mice by blocking crypt epithelial apoptosis and promoting epithelial cell survival and self-renewal through upregulation of stem cell factors (LGF5, BMI-1, Hopx, mTERT, Lrig1) and inhibiting radiation induced P53 apoptotic response.16,17,18,19,24 Prophylactic therapy with various growth factors and cytokines such as TGFb, IL-11, keratinocyte growth factor (KGF), and Kruppel-like factor (KLF) increases crypt survival following radiation.1,2,7,15 Radiation induced injury to the intestinal tract is also associated with increased levels of oxidative tissue injury, and studies have shown that certain antioxidants and mediators of oxidative stress play a role in RIGS and its progression. For example, cyclooxygenase induced prostaglandins prevent cytotoxicity of crypt epithelial cells in mice,16 and alterations in the mTOR-PI3K and NRF2 pathways regulating oxidative stress can protect the intestinal tract through mediation of oxidative stress and upregulation of stem cell factors and growth pathways such as the NF-kB pathway.5,25 Finally, blockage of the TLR pathway, important in promotion of inflammatory responses to microbial pathogens, protects from lethal radiation induced intestinal injury in mice.22
Since intestinal stem cells are a target of radiation induced toxicity, activation of developmental and stem-cell embryonic pathways have shown to be effective in mediating RIGS in animal models, as epithelial regeneration following radiation injury requires intestinal stem cell repopulation.12,14,19 For example, activation of the Wnt/beta-catenin pathway or suppression of the adenomatous polyposis coli (Apc) gene function in radiation injury stimulates repair and significantly improves morbidity and mortality in mice following high dose radiation.19 Notch signaling, another developmental/stem cell pathway, regulates self-renewal of intestinal stem cells and activation of this pathway accelerates reversal of radiation induced damage in the intestinal tract.14 Mesenchymal stem cells, or stromal progenitor cells (SPCs) have been shown to have regenerative, immune modulatory, angiogenic, and anti-inflammatory properties that promote healing.3,21 In addition, the recruitment of extra-intestinal and bone marrow-derived cells has been shown to play a role in promotion of intestinal healing and improvement of morbidity and mortality in radiation-induced intestinal injury through secretion of growth factors, stem cell mediators, and inflammatory cytokines in a paracrine fashion.21
Lastly, there is a significant role of the microbiome in the response to RIGS.8,13 Radiation of the intestinal tract results in alterations in normal intestinal microbiota, which is an important factor in the pathogenesis of radiation enteritis; radiation reduces the normal diversity of the gut microbiota and leads to dysbiosis, which aggravates RIGS by weakening intestinal epithelial barrier function and promoting inflammation.4,9 Changes in the microbiome of the intestine reflect altered diversity reflective of decreased beneficial Lactobacillus and Bacterioides spp. and increased E. coli and Streptococcus spp.23,26 As a further result of altered diversity of microbes, the composition of short-chain fatty acids (SCFAs), the key metabolites generated by large intestinal microbial metabolism, is altered as well; SCFAs play an important role in intestinal repair, inflammation, and homeostasis in the gut.26 Transplantation of normal fecal microbiota has been shown to improve survival in irradiated animals and improve overall function of the intestinal tract.4 Dysbiosis also promotes inflammation and alterations in barrier function in the intestinal tract, influenced by enhanced pro-inflammatory cytokine signaling through TNFa and IL1b expression, and rearrangement of important tight junction proteins induced by microbes such as pathogenic E. coli.9,23 In fact, beneficial effects on intestinal symptoms have been observed using probiotics including Lactobacillus in animal models and humans in reducing endotoxin levels, reduction in severity of bowel injury, and reduction in potential bacteremia.13
In summary, a full understanding of the pathophysiology of radiation induced injury in the intestinal tract is still incomplete, but likely involves a complex interplay between epithelial damage and repair, induction of developmental and stem cell pathways, growth factors and cell cycle/DNA damage mediators, the inflammasome, endothelial injury, tissue remodeling, and effects on the enteric nervous system, influenced by alterations in normal gut microbiota to result in the clinical syndrome of RIGS.
Contributing Institution:
In Vivo Animal Core, Unit for Laboratory Animal Medicine
University of Michigan Medicine
North Campus Research Complex, Building 36, Rm G177
2800 Plymouth Road
Ann Arbor, MI 48109
JPC Diagnosis:
- Colon: Colitis, necrotizing, multifocal to coalescing with glandular regeneration and lymphoid depletion.
- Small intestine: Enteritis, necrotizing, segmental, moderate, with ulceration, crypt abscesses, and crypt hyperplasia.
JPC Comment:
The contributor provides an excellent description of radiation-induced gastrointestinal syndrome. To see the effects of ionizing radiation on the lung, WSC 2021, Conference 17, Case 4 is a case of radiation pneumonitis in a rhesus macaque exposed to whole thorax radiation, and the contributor described the pathogenesis behind acute and chronic phases of injury.
This week?s moderator, Dr. Cory Brayton of Johns Hopkins University School of Medicine, commented on the atypical features of regeneration in this case, including anyisocytosis, anisokaryosis, and presence of goblet cells in the crypts, which provide clues as to pathogenesis of this case. IBA-1 revealed the scattered inflammatory infiltrate in the lamina propria to be composed of abundant macrophages called into clean up necrotic cellular debris.
Additionally, she pointed out a histoanatomic feature which is helpful identifying origin of a colonic section: the mucosal folds in present in the colonic section in this case are more prominent in the proximal section of the colon and correspond to grossly visible diagonal lines on the serosal surface.
Acute radiation syndrome occurs after exposure to a substantial amount of ionizing radiation and may occur as a result of radiotherapy/radiopharmaceuticals, nuclear accidents, or atomic bombings. The effects are dependent on both the dose and type of radiation and regions and proportion of the body exposed. Systems particularly sensitive to acute radiation injury include the hematopoietic, gastrointestinal, integumentary, and nervous systems. The prodrome phase of ARS occurs immediately after exposure and results in nausea, vomiting, fatigue, or loss of consciousness from autonomic stability.6 This is followed by a variable and dose-dependent latent period followed by manifest illness.6 Apoptosis of myeloid precursors in bone marrow and peripheral leukocytes (particularly lymphocytes) leads to cytopenias.6,11 Thrombocytopenia results in hemorrhage, while lymphopenia and neutropenia impair immune function.6,11 Radiation induced gastrointestinal system leads to transmigration of bacteria and subsequent septicemia.6 At higher levels of exposure, neurologic injury and hemorrhage injury occurs.6,11
The radiation exposure level required to induce clinical signs is variable between species and between individuals. The LD50/30, or the dose where 50% of animals survive 30 days without medical care, varies from as little as 2.5 Gy in swine to up to 10 Gy in Mongolian gerbils.6 For reference, 1 Gy is approximately 300 times the annual background radiation exposure of a person in the US.20
Severity of radiation injury is compounded when it is followed by or simultaneous with other trauma (i.e. thermal burns, wounds).6,11 In a canine study, thermal burns covering 20% of the body resulted in minimal mortality, but when combined with 1 Gy exposure, burns of the same extent caused 73% mortality.6 This compounded effect is attributed, at least in part, to delayed wound healing and acute immunosuppression from radiation injury.6,11 The likelihood of polytrauma in radiation exposure secondary to nuclear weapon detonation is high: approximately 70% of atomic bomb survivors in Hiroshima and Nagasaki and 10% of Chernobyl nuclear accident survivors experienced combined injuries.11
Effects of acute radiation syndrome are considered deterministic, as exposure above a certain threshold produces these injuries consistently as a result of direct cellular damage and tissue reactions.10 Deterministic effects may occur acutely or have a late onset.10 Stochastic effects, on the other hand, tend to have a late onset and are the result of genetic damage. These do not occur as consistently as deterministic effects; rather, the incidence of disease increases proportionally with the degree of exposure. An example of a stochastic effect is an increased risk of cancer due to mutations in somatic cell DNA; hereditary effects also occur as a result of mutations in germline cells.10
Long term stochastic effects of radiation exposure have been extensively documented in the Life Span Study, a decades-long and ongoing study evaluating the medical outcomes of 93,741 atomic bombing survivors from Hiroshima and Nagasaki and 26,580 unexposed cohorts.20 The earliest stochastic effect was an increase in rates of leukemia seen within two years of the bombings. Since then, the study has uncovered a significant linear and dose dependent increase in the occurrence of cancers in multiple locations, including the stomach, lung, liver, colon, thyroid, and skin.20 Risks of death due to solid cancers, stroke, and heart disease are significantly increased in exposed individuals.20 The study also found a decrease rate of growth in those who were 5-15 years old at the time of the bombings.20
References:
- Boerma M, Wang J, Sridharan V, Herbert JM, Hauer-Jensen M. Pharmacological induction of transforming growth factor-beta1 in rat models enhances radiation injury in the intestine and the heart. PLoS One. 2013;8:
- Booth C, Tudor G, Tudor J, Katz BP, MacVittie TJ. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012;103: 383-399.
- Chang PY, Zhang BY, Cui S, et al. MSC-derived cytokines repair radiation-induced intra-villi microvascular injury. Oncotarget. 2017;8: 87821-87836.
- Cui M, Xiao H, Li Y, et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol Med. 2017;9: 448-461.
- Datta K, Suman S, Fornace AJ, Jr. Radiation persistently promoted oxidative stress, activated mTOR via PI3K/Akt, and downregulated autophagy pathway in mouse intestine. Int J Biochem Cell Biol. 2014;57: 167-176.
- DiCarlo AL, Maher C, Hick JL, et al. Radiation Injury After A Nuclear Detonation: Medical Consequences and the Need for Scare Resources Allocation. Disaster Med Public Health Prep. 2011; 5 Suppl 1: S32-44.
- Farrell CL, Bready JV, Rex KL, et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 1998;58: 933-939.
- Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy--pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol. 2014;11: 470-479.
- Jian Y, Zhang D, Liu M, Wang Y, Xu ZX. The Impact of Gut Microbiota on Radiation-Induced Enteritis. Front Cell Infect Microbiol. 2021;11:
- Kamiya K, Ozasa K, Akiba S, et al. Long-term effects of radiation exposure on health. Lancet. 2015; 386: 469-478.
- Kiang JG, Olabisi AO. Radiation: a poly-traumatic hit leading to multi-organ injury. Cell Biosci. 2019; 9(25):1-15.
- Kulkarni S, Wang TC, Guha C. Stromal Progenitor Cells in Mitigation of Non-Hematopoietic Radiation Injuries. Curr Pathobiol Rep. 2016;4: 221-230.
- Kumagai T, Rahman F, Smith AM. The Microbiome and Radiation Induced-Bowel Injury: Evidence for Potential Mechanistic Role in Disease Pathogenesis. Nutrients. 2018;10.
- Kwak SY, Shim S, Park S, et al. Ghrelin reverts intestinal stem cell loss associated with radiation-induced enteropathy by activating Notch signaling. Phytomedicine. 2021;81:
- Li M, Gu Y, Ma YC, et al. Kruppel-Like Factor 5 Promotes Epithelial Proliferation and DNA Damage Repair in the Intestine of Irradiated Mice. Int J Biol Sci. 2015;11: 1458-1468.
- MacNaughton WK. Review article: new insights into the pathogenesis of radiation-induced intestinal dysfunction. Aliment Pharmacol Ther. 2000;14: 523-528.
- Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14: 149-159.
- Pant V, Xiong S, Wasylishen AR, et al. Transient enhancement of p53 activity protects from radiation-induced gastrointestinal toxicity. Proc Natl Acad Sci U S A. 2019;116: 17429-17437.
- Romesser PB, Kim AS, Jeong J, Mayle A, Dow LE, Lowe SW. Preclinical murine platform to evaluate therapeutic countermeasures against radiation-induced gastrointestinal syndrome. Proc Natl Acad Sci U S A. 2019;116: 20672-20678.
- Sakata R, Grant EJ, Ozasa K. Long-term follow-up of atomic bomb survivors. Maturitas. 2012; 72: 99-103.
- Sung J, Sodhi CP, Voltaggio L, et al. The recruitment of extra-intestinal cells to the injured mucosa promotes healing in radiation enteritis and chemical colitis in a mouse parabiosis model. Mucosal Immunol. 2019;12: 503-517.
- Takemura N, Kawasaki T, Kunisawa J, et al. Blockade of TLR3 protects mice from lethal radiation-induced gastrointestinal syndrome. Nat Commun. 2014;5:
- Wang Z, Wang Q, Wang X, et al. Gut microbial dysbiosis is associated with development and progression of radiation enteritis during pelvic radiotherapy. J Cell Mol Med. 2019;23: 3747-3756.
- Wei L, Leibowitz BJ, Wang X, et al. Inhibition of CDK4/6 protects against radiation-induced intestinal injury in mice. J Clin Invest. 2016;126: 4076-4087.
- Yang W, Sun Z, Yang B, Wang Q. Nrf2-Knockout Protects from Intestinal Injuries in C57BL/6J Mice Following Abdominal Irradiation with gamma Rays. Int J Mol Sci. 2017;18.
- Zhu S, Liang J, Zhu F, et al. The effects of myeloablative or non-myeloablative total body irradiations on intestinal tract in mice. Biosci Rep. 2021;41.