CASE 3: N-361/19 (4156029-00)
Signalment:
Adult, female, striped dolphin (Stenella coeruleoalba)
History:
The dolphin was found swimming in circles at 100 meters from the seaside, in Tamariu, Girona (Catalonia). The external examination revealed marked dehydration and increased heart and breathing rates. One hour later, the dolphin started to show seizures, and due to the poor prognosis, she was humanly euthanized and submitted for a complete post-mortem examination.
Gross Pathology:
The dolphin (length: 175 cm, weight: 56.5 Kg) was well preserved and had a moderate loss of body condition. External parasites were not present. No ingesta was found in the forestomach, and the secretory stomach was empty. No other relevant gross lesions were found. Common endoparasites of the species, such as Phyllobothrium delphini and Monorygma grimaldii larval forms, were found in low numbers.
Laboratory results:
- RT-PCR Cetacean Morbillivirus (CeMV):
- CNS (brain cortex) positive.
- Lymph-node, lung and spleen: negative.
- Immunohistochemistry (IHC) CeMV:
- brain cortex: positive.
- Lung, lymph node, spleen: negative.
Microscopic description:
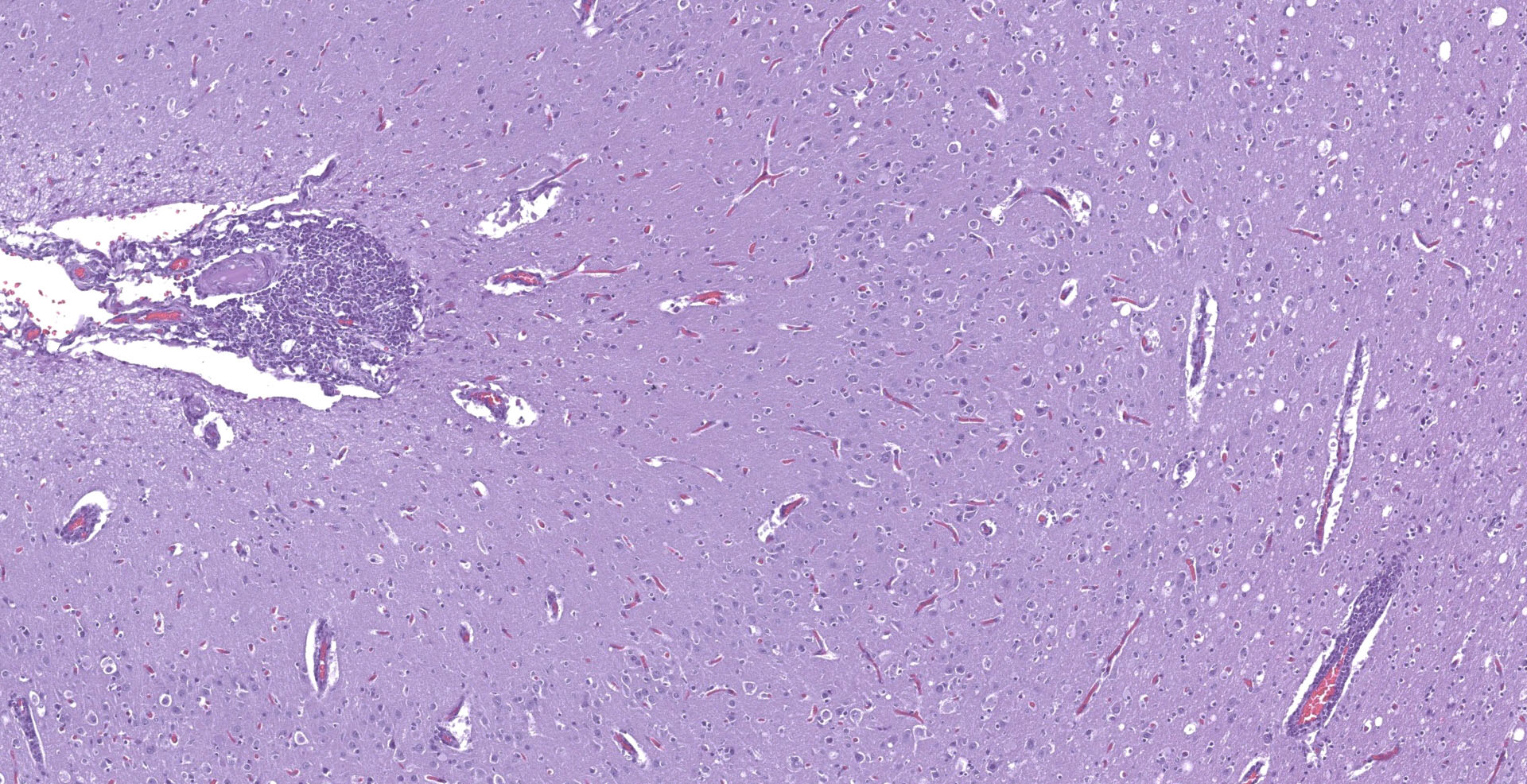
With a patchy pattern throughout the cerebral cortex, and specially the left hemisphere, there is a severe chronic inflammatory process mainly located in the grey matter. Due to this uneven distribution, the microscopic lesions described might not be present in all the slides.
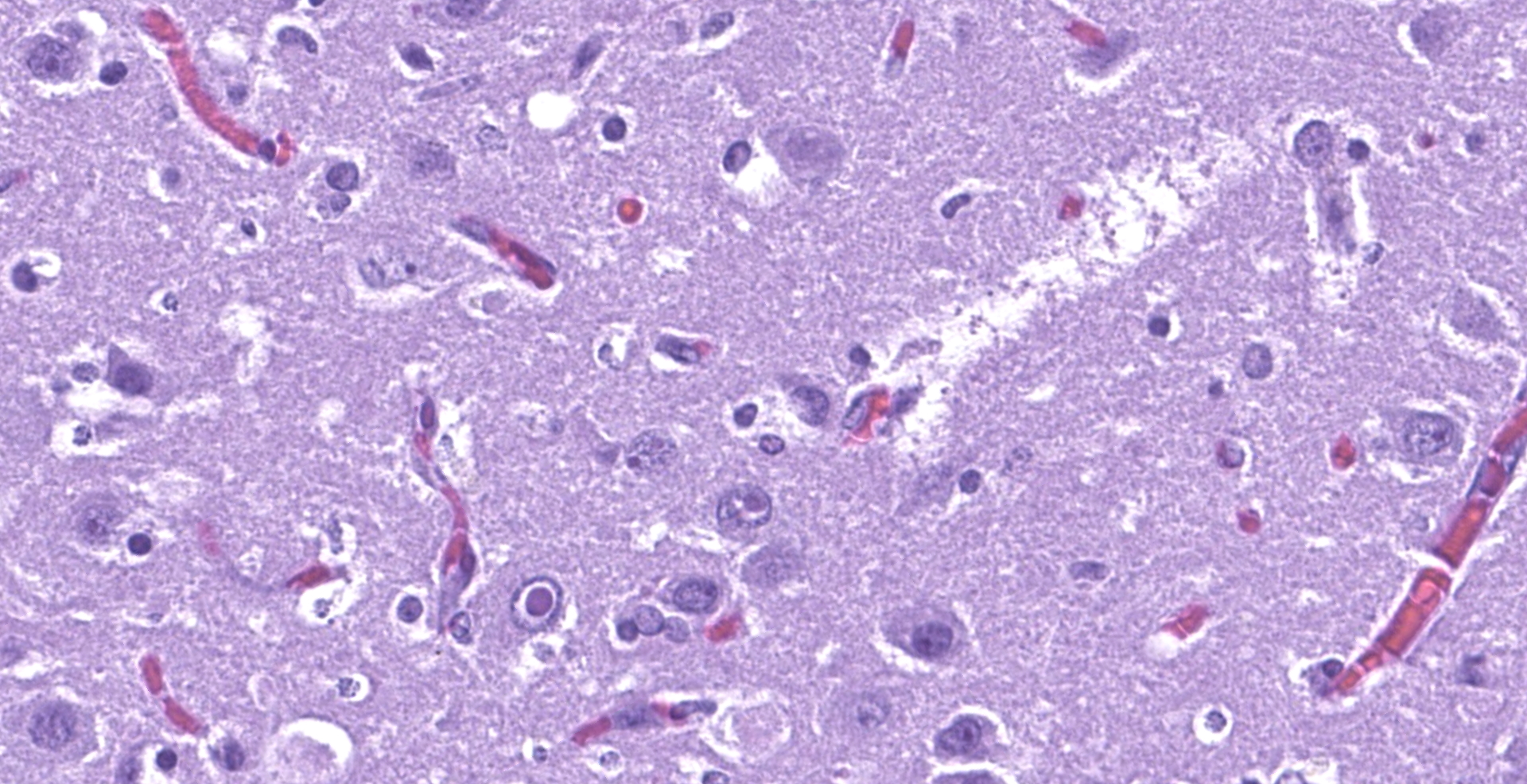
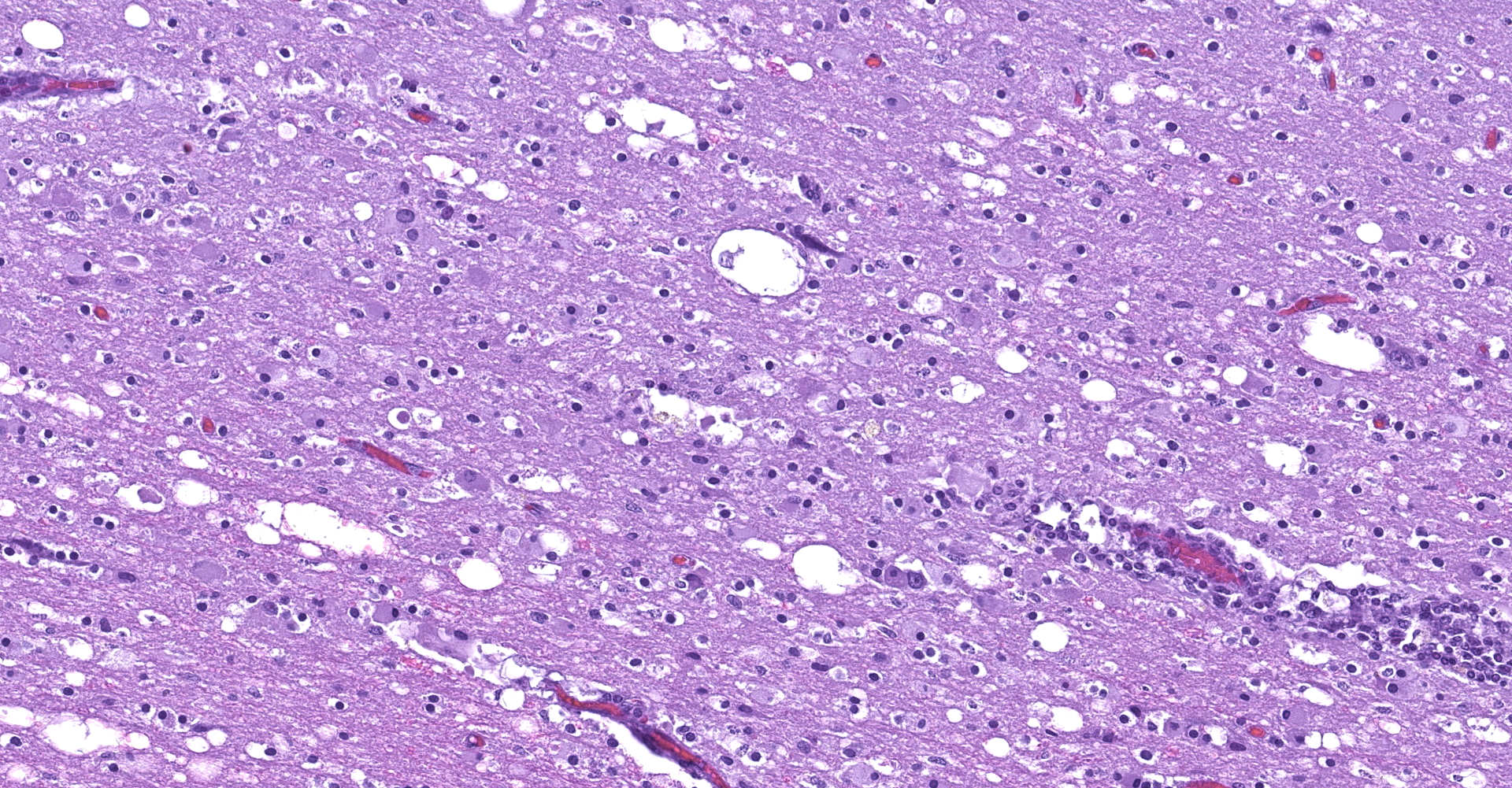
The affected areas of the grey matter show a mild to moderated distortion of the normal texture and architecture of the neuropil due to vacuolization (spongiosis) and loss of neuronal bodies, which are replaced by aggregates of activated astrocytes and microglia (glial foci). Numerous of the remaining neurons show margination of the Nissl granules to the periphery (central chromatolysis) or different features of degeneration and necrosis such as a shrunken and hypereosinophilic cytoplasm, pyknosis and loss of the cell nucleus. These neurons are surrounded by activated phagocyting microglia (neuronophagy) or glial cells (satellitosis). Within the nucleus of many of these neurons, there is a single round, 2 to 7 micrometers in diameter, pale eosinophilic viral inclusion.
Multifocally, the meninge and Virchow-Robin spaces are expanded by up to 10 cells thick mononuclear perivascular cuffing composed of lymphocytes, plasma cells and occasional histiocytes. There is also moderate perivascular edema which extends towards the adjacent neuropil.
Multifocal areas of spongiosis, astrocytosis and astrogliosis can be seen in the white matter.
Contributor's morphologic diagnosis:
Brain (left cortex). Multifocal, chronic, severe mononuclear (lymphoplasmacytic, histiocytic) meningoencephalitis with nuclear viral inclusions.
Contributor's comment:
Cetacean morbillivirus (CeMV) was first reported in 1990 in the Western Mediterranean causing a mass mortality in striped dolphins.4 There are at least three well characterized strains distributed worldwide, including the porpoise morbillivirus (PMV), dolphin morbillivirus (DMV) and pilot whale morbillivirus (PWMV). Recently, new strains have been also described in a Longman's beaked whale (Indopacetus pacificus) from Hawaii, in a Guiana dolphin (Sotalia guianensis) from Brazil, and in Indo-Pacific bottlenose dolphins (Tursiops aduncus) from Western Australia.1 CeMV is a relevant cause of mortality cetaceans, causing pneumonia, lymphoid depletion, and encephalitis, as well as immunosuppression and facilitation of secondary opportunistic infections.5 Morbillivirus transmission is thought to occur mainly by inhalation of aerosolized virus shed by infected animals, but transplacental transmission has also been reported. Moreover, transmission between groups is facilitated due to the migratory behavior.
After infection by CeMV, there is an initial replication in lymphoid tissues and a subsequent systemic dissemination, reaching other cell types, mainly epithelia. As a result of lymphoid depletion, there is a severe immunosuppression, hence secondary infections are a common finding in CeMV-affected dolphins. Although CeMV can be pancytopathic, the main damaged cells are those of the respiratory, lymphoid and central nervous system.13
In the Mediterranean Sea, CeMV has caused two well-documented mass mortalities in 1990?1992 and 2006?2008, mainly in striped dolphins, but also in long finned pilot whales (Globicephala melas).6,9 During the interepizootic period, all the necropsied animals in the Catalonian coast were immunohistochemically negative for CeMV, hence it was assumed that CeMV was not circulating in that period.11
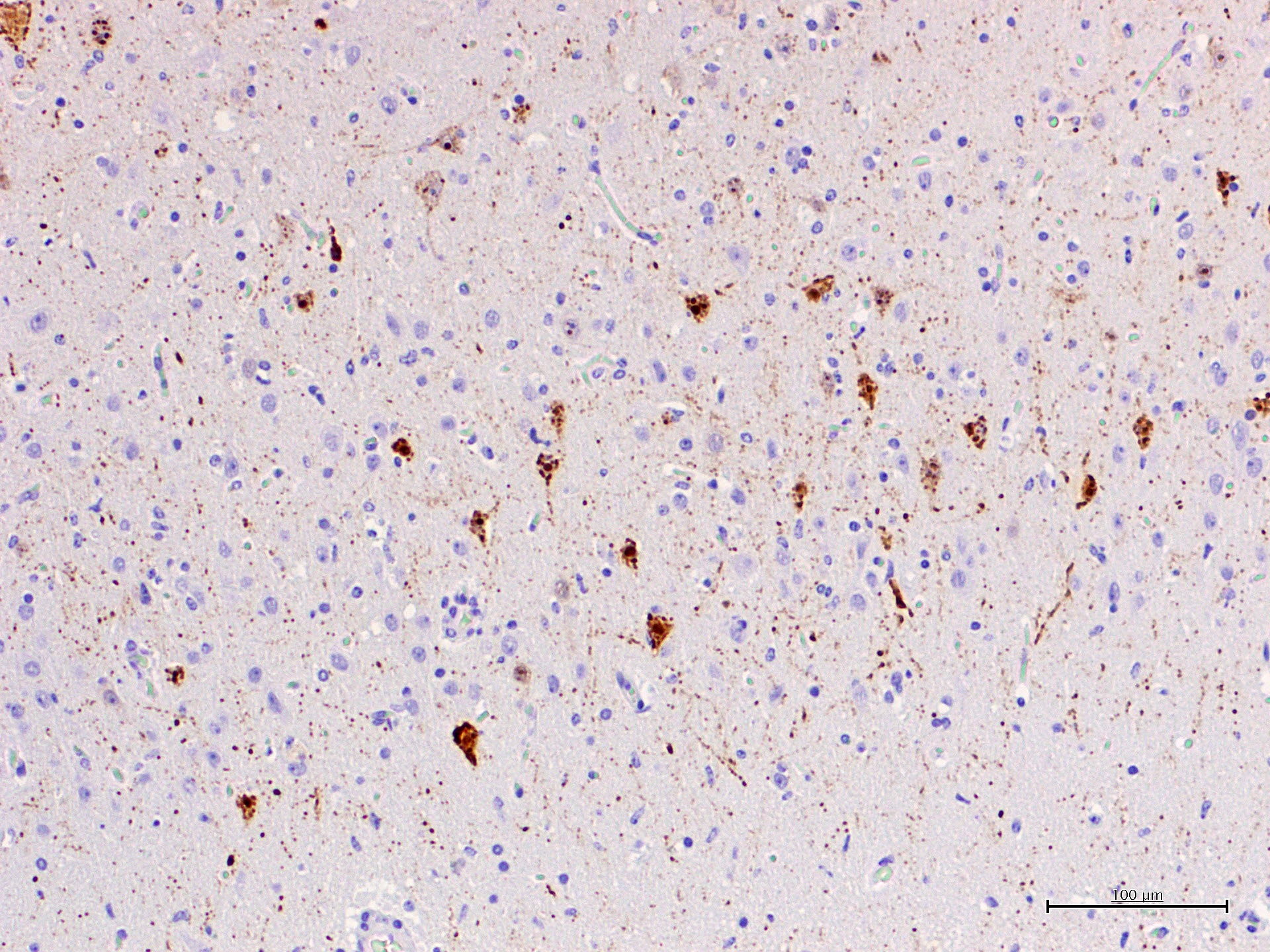
CeMV circulation and death of dolphins also occurred in 2012 and 2016-2017 in the same area, although apparently with a much lower mortality. In the four years after the two main epizootic waves, cases with exclusive affection of the central nervous system were observed and were referred as chronic CNS infections.12 These cases were interpreted as reactivation of a chronic infection, similarly to subacute sclerosing panencephalitis (SSPE) in humans or old dog encephalitis (ODE) in dogs, but a definitive proof is lacking. Similar encephalitis cases have been observed after a recirculation of CeMV in striped dolphins in the period 2016-2017. The submitted case is one of these cases. What is peculiar of these encephalitis cases of is the absence of lesions and CeMV antigen or its genomic material outside the CNS. Viral antigen, demonstrated with IHC against the N-protein with a MoAb raised against CDV (VMRD) shows accumulation of antigen in cell body and processes of neurons, often without evidence of clear cytopathic effect.
Summarizing, different forms of CeMV infection may be observed:1
- Acute systemic disease
Characterized by severe multifocal to diffuse interstitial pneumonia with necrosis of bronchiolar epithelium, formation of large syncytia and nuclear viral inclusions in syncytia and epithelial cells of the lung. There is also diffuse lymphoid depletion with germinal center necrosis, with syncytial cells. Multifocal non-suppurative encephalitis may also be present. In such cases, there is a strong immunohistochemical (IHC) staining in the organs affected.
- Sub-acute systemic disease
Animals that survive the acute infection and present secondary infections such as aspergillosis, Toxoplasma gondii, or herpesviruses. The lesions of the acute form may not be present or can be obscured by these secondary processes. Both IHC and RT-PCR are useful for confirmation of the diagnosis.
- Chronic, localized encephalitis
This form is hypothesized to be the result of clearing the initial systemic infection and of reactivation restricted to the CNS, with absence of lesions and antigen outside of this organ. In these cases, there are occasional nuclear viral inclusion and no syncytia. Besides, there is a strong IHC staining of the neuronal processes with a patchy pattern, mixing areas of high-antigen content with others free of antigen. A cell-to-cell spread of infection is suggested for these cases, rather than a multifocal infection indicative of blood-borne infection. This form shares multiple characteristics with subacute sclerosing panencephalitis (SSPE) and old dog encephalitis (ODE), which are chronic latent localized infections affecting humans and dogs. The most prominent lesions are perivascular cuffing, diffuse gliosis and glial nodules with neuronophagia. Demyelination is less prominent in dolphins compared with ODE. In these cases, IHC and RT-PCR for CeMV are also helpful tools to reach a final diagnosis.
- Subclinical infection
The existence of a subclinical CeMV infection in cetaceans has been suggested but remains speculative. These subclinical infections would help to disseminate the virus to long distances.
The present case is a florid example of a chronic localized encephalitis, with many evident eosinophilic nuclear viral inclusions. The multifocal pattern is one of the characteristics of this stage, as well as the strong IHC staining of the neuronal processes also observed in this dolphin. As expected in such cases, no other lesions were found in the lymphoid and respiratory system. Furthermore, only the CNS sample resulted positive to CeMV RT-PCR and IHC (lung, lymph-node and spleen were negative).
Contributing Institution:
Veterinary Pathology Department
Autonomous University of Barcelona
Barcelona, Spain
https://www.uab.cat/web/els-serveis/-servei-diagnostic-de-patologia-veterinaria-1297063220061.html
JPC diagnosis:
Cerebrum: Meningoencephalitis, lymphohistiocytic and necrotizing, multifocal to coalescing, moderate, with gliosis, spongiosis, and intracytoplasmic and intranuclear viral inclusions.
JPC comment:
The contributor provides a good summary of Cetacean morbillivirus as we understand it today. There was discussion about which cells contain the viral inclusions. Participants had a reasonable degree of certainty that both neurons and astrocytes contained viral inclusions, and that the perivascular lymphocytes may also have contained viral inclusions.
Morbilliviruses use two cellular receptors, signaling lymphocyte activation molecule (SLAM), and nectin-4. Unlike nectin-4, which is highly conserved across species, the SLAM/CD150 molecule is more divergent and specific to different species of hosts. The structure of SLAM is based around 35 residues that allow it to bind to the viral H protein. The 35 residues of interest are conserved between pinnipeds and are highly similar to the residues of the suborder Caniformia (dogs, otters, mink), suggesting a potentially large community for which both canine distemper virus and phocine distemper virus may infect. The set of 35 residues is more divergent in cetaceans, potentially suggesting an encoded H protein with broader binding specificity to host receptors in these populations.8
There is evidence that this virus is capable of cross-species transmission, with the Dolphin morbillivirus (DMV) strain's ability to use both dolphin and seal SLAM/CD150 as host cell receptors. Using Bayesian phylogeographic analysis, CeMV is estimated to have a mutational rate of 2.34 x 10-4 nucleotide substitutions/site/year, regardless of host or geographic region.7 The rinderpest virus (or of closely related cattle origin) is believed to have crossed the bovine-human barrier 1000-5000 years ago, leading to an offshoot that became the measles virus. A common ancestor between rinderpest virus and cetacean morbillivirus has been hypothesized, though it may be equally likely that CeMV originated directly from rinderpest virus. The ability for these viruses to cross species increases the likelihood that new morbillivirus species will continue to evolve and affect animals, both on land and sea.3
Knowledge gaps still exist in our understanding of this virus and disease. While some receptor interactions are relatively well characterized, characterization of cell receptors in the central nervous system may help define its neurotropism. We also do not yet know the magnitude or contribution from environmental contaminants on the host's manifestation of disease. Toxins like polychlorinated biphenyls, methylmercury, dioxins, and others, accumulate in long lived marine animals as a process of bioaccumulation (also termed biomagnification). We do not yet know how predominantly Th1 response differs from predominately Th2 response in the host. Different characterization of host immune response is critical for the development of many known disease, such as AIDS in humans. Similarly, CeMV has been identified in newborns and fetuses. The host immune response is often operating at reduced efficiency during pregnancy, and we do not yet understand what effect that may have on the development of disease.2
During the case discussion, a pathologist raised the possibility that this striped dolphin had a co-infection that worsened the meningoencephalitis. A recent study of viral, bacterial, protozoal, and metazoan causes of cetacean meningoencephalitis, viral etiologies (CeMV, Herpesvirus) were the most common cause, but generally only resulted in minimal or mild non-suppurative meningitis. However, the animals that presented with more severe cases of meningitis had a coinfection with Brucella sp. Unfortunately, a culture was not performed in this case.10
References:
1. Van Bressem MF, Duignan PJ, Banyard A, et al. Cetacean morbillivirus: Current knowledge and future directions. Viruses. 2014;6:5145?5181.
2. Di Guardo G, Centelleghe C, Mazzariol S. Cetacean Host-Pathogen Interaction(s): Critical Knowledge Gaps. Frontiers in Immunology. 2018;9:02815.
3. Di Guardo G, Mazzariol S. Cetacean morbillivirus: A Land-to-Sea Journey and Back? Virologica Sinica. 2019;34:240-242.
4. Domingo M, Ferrer L, Pumarola M, et al. Morbillivirus in dolphins [7]. Nature. 1990;348:21.
5. Domingo M, Visa J, Pumarola M, et al. Pathologic and immunocytochemical studies of morbillivirus infection in striped dolphins (Stenella coeruleoalba). Vet Pathol. 1992;29:1?10.
6. Fernández A, Espero F, Herraéz P, et al. Morbillivirus and pilot whale deaths, Mediterranean Sea. Emerg Infect Dis. 2008;14:792?794.
7. Jo WK, Kruppa J, Habierski A, et al. Evolutionary evidence for multi-host transmission of cetacean morbillivirus. Emerging Microbes and Infections. 2018;7:201.
8. Ohishi K, Maruyama T, Seki F, Takeda M. Marine Morbilliviruses: Diversity and Interaction with Signaling Lymphocyte Activation Molecules. Viruses. 2019;11(7):606.
9. Raga JA, Banyard A, Domingo M, et al. Dolphin morbillivirus epizootic resurgence, Mediterranean Sea. Emerg Infect Dis. 2008;14:471?473.
10. Sierra E, Fernandez A, Felipe-Jimenez I, et al. Histopathological differential diagnosis of meningoencephalitis in cetaceans: Morbillivirus, Herpesvirus, Toxoplasma gondii, Brucella sp., and Nasitrema sp. Frontiers in Veterinary Science. 2020;7:650.
11. Soto S, Marco A, Domingo M, et al. Epizootic of dolphin morbillivirus on the Catalonian Mediterranean coast in 2007. Vet Rec. 2011;169.
12. Soto S, Alba A, Ganges L, et al. Post-epizootic chronic dolphin morbillivirus infection in Mediterranean striped dolphins Stenella coeruleoalba. Dis Aquat Organ. 2011;96:187?194.
13. St Leger J, Raverty S, Mena A. Cetacea. In: Pathology of Wildlife and Zoo Animals. Elsevier; 2018:533?568.