CASE 1: 200720011 (4152712-00)
Signalment:
A 57.4 lb, adult, female, tan and white kangaroo (Macropus sp.) in good body condition.
History:
Kangaroos in an exhibit were stung by a swarm of honeybees (Apis mellifera). Several kangaroos
died over the next 4 days.
Gross Pathology:
At necropsy, there was marked facial swelling, more prominent over the right mandible, and diffuse icterus. The lungs were rubbery and meaty with red to dark red coalescing areas. The liver was diffusely yellow with an accentuated reticular pattern.
Laboratory results:
N/A
Microscopic description:
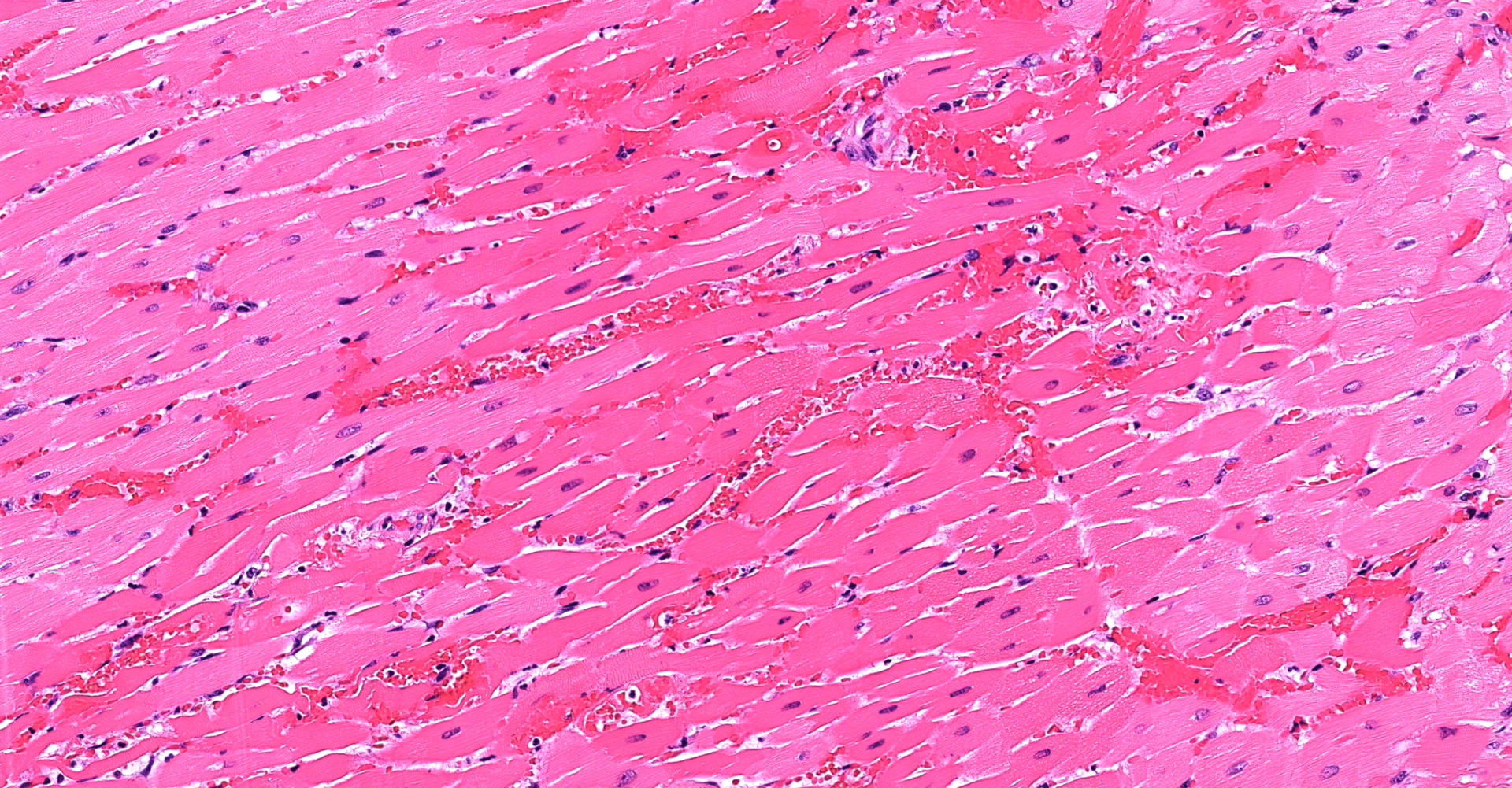
Heart (left ventricle papillary muscle and myocardium): The subendocardial myocardium has multifocal areas of acute coagulative necrosis (more prominent in other sections) accompanied by moderate hemorrhage. Within these areas, and to a lesser extent deeper in the myocardium, cardiomyocytes exhibit variable swelling, sarcoplasmic hypereosinophilia, vacuolation, myofibrilysis, fragmentation and/or loss of cross striations. Early neutrophilic infiltration and pyknosis/karyorrhexis/apoptosis of leukocytes, interstitial cells, and cardiomyocytes are noted. In affected areas, capillary walls are disrupted and may have lost their endothelial lining and wall detail. The interstitium is expanded by clear space, light basophilic fluid and eosinophilic globules (serous fluid, myoglobin).
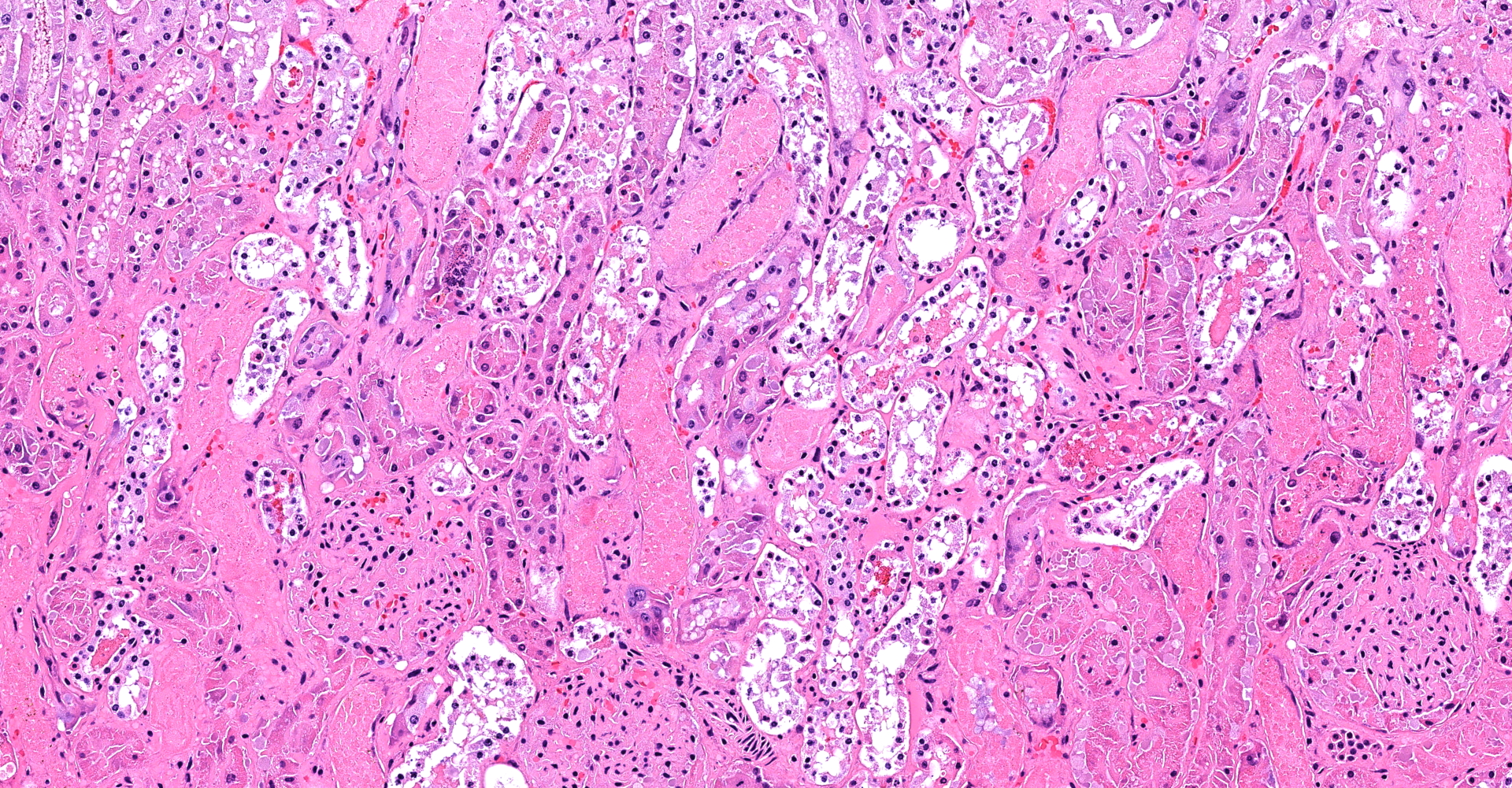
Kidney (cortex and medulla): Despite some postmortem autolysis and decomposition phenomena, diffusely, tubuloepithelial cells of cortical and medullary convoluted tubule segments exhibit acute degenerative and necrotic changes. These include attenuation, swelling, vacuolation, hypereosinophilia, pyknosis/karyorrhexis/apoptosis, partial preservation of cellular outlines and detachment from the underlying tubular basement membrane. Some of these tubules show tubulorrhexis. Affected lumens are often dilated and contain a combination of cellular and proteinaceous casts and red granular material (hemoglobin/myoglobin). Few tubule segments show early regenerative attempts including ample polygonal epithelial cells with large nuclei, prominent nucleoli and rare mitotic figures. Multifocal acute hemorrhage and hypereosinophilic fluid expand the interstitium.
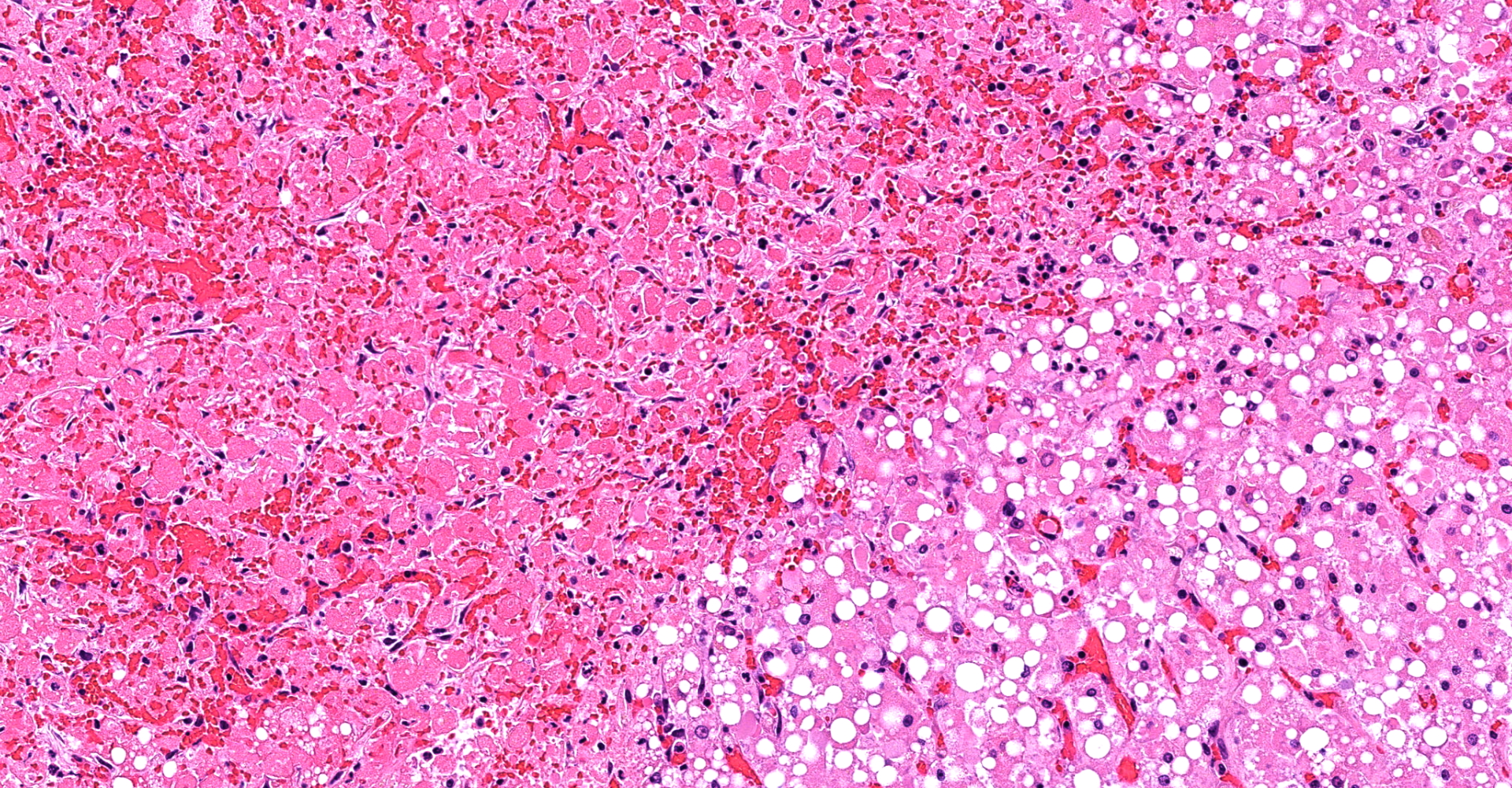
Liver (including Glisson's capsule): Multiple subcapsular hepatic lobules show diffuse acute coagulative necrosis and hemorrhage. In these areas, hepatocytes exhibit cytoplasmic vacuolation, hypereosinophilia, pyknosis/karyorrhexis/apoptosis, cell disruption, dissociation and/or loss. Also, there is early neutrophilic infiltration with fibrin and edema. Few bile duct profiles remain and contain scarce early oval cell hyperplasia/ductular metaplasia are noted. In adjacent lobules, hepatocytes exhibit prominent macro- and microvacuolar change (lipid type), more severe in midzonal to centrilobular areas. Smaller discrete clusters or single hepatocyte necrosis and hemorrhage are noted in these areas. Sinusoids are collapsed and there is loss of the space details. Some terminal plates and subterminal hepatocytes are of Disse and endothelial profile relatively preserved.
Contributor's morphologic diagnosis:
1. Heart: Marked, multifocal, acute myocardial coagulative necrosis with hemorrhage.
2. Kidney: Marked, diffuse, acute tubuloepithelial degeneration and necrosis with tubular proteinosis, hemoglobin/myoglobin casts and hemorrhage.
3. Liver: Marked, multifocal, acute hepatic necrosis with hemorrhage.
Contributor's comment:
Based on the clinical history and the pathologic findings, fatal "bee massive envenomation" (so called "systemic toxic reaction") was determined as the cause of death in this kangaroo.14 Reasonable differential diagnoses in this case may include: infectious diseases e.g., toxoplasmosis and acute toxicities e.g., mebendazole toxicosis (previously associated with "hemorrhagic septicemic syndrome", anticoagulant poisoning, copper toxicosis, Lantana camara toxicosis, snake envenomation, among others.20 Visual confirmation of honeybee attack and the cutaneous changes observed over the right mandible were crucial components to reach a final diagnosis.
The Apoidea (bees) and Vespoidea (wasps, hornets, and yellow jackets) are among the most medically relevant hymenoptera worldwide. The incidence of bee stings has increased over the last decades and are regarded a public health concern throughout most of the Americas.17 Given that the stinging events are rarely witnessed, and the pathologic findings are non-specific,7 fatal bee envenomation other than in humans and dogs is likely underreported. Few cases involving horses and sheep have been published.15,18,19 Most hymenopteran stings are self-limiting events albeit in a number of cases, victims may experience immediate life-threatening anaphylactic reactions. In humans, the estimated lethal dose ranges between 2.8 and 3.5 mg of venom per kg of body weight.12 Noteworthy, anaphylactic reactions to Hymenoptera stings are
not volume-dependent (or related to the number of stings). This is opposed to the direct toxic effects (volume-dependent) exerted by some of the venom's components. Indeed, the severity of the envenomation is determined by a combination of the victim's age, body weight, number of stings, and other individual characteristics of the victim such as the immune status, comorbidities, and previous sensitization.12
Bee venom is a complex mixture of compounds that includes proteins, peptides, amino acids, phospholipids, sugars, biogenic amines, volatile compounds, pheromones, and water.4 The main components with clinicopathological relevance are melittin, phospholipase A2 (PLA2), apamin, hyaluronidase, mast cell degranulating peptide (MCD), vasoactive amines (e.g., histamine, dopamine, and norepinephrine), phospholipases B (PLB), serotonin, adolapin, acid phosphatase, and minimine. Briefly, melittin is a lytic peptide that is able to disrupt membrane phospholipids. Phospholipase A2 acts in concert with melittin ("bee hemolytic factor") to cause intravascular hemolysis. Apamin is a neurotoxin that selectively inhibits Ca2+-dependent K+ channels, with potent action on the central nervous system. Hyaluronidase ("spreading factor") alters cell permeability and disrupts collagen, thereby, allowing other venom components to penetrate into the victim's tissues. MCD causes mast cells to degranulate, releasing histamine and vasoactive amines; however, in high quantities, it may be anti-inflammatory.12
Clinically, bee envenomation may be divided into 1) local inflammatory reactions; 2) allergic manifestations; 3) anaphylactic shock; 4) delayed hypersensitivity reaction, and 5) systemic toxic reactions (so called "massive envenomation" or "envenoming syndrome").6 Most deaths related to hymenoptera stings are the direct result of the immediate hypersensitivity reaction. In sensitized individuals (previous contact with bee venom), IgE antibodies attach to tissue mast cells and basophils causing their degranulation with subsequent release of vasoactive substances, leukotrienes, histamine, and eosinophil chemotactic factor-A. In anaphylactic reactions, death may ensue within several minutes. Furthermore, some deaths are attributed to severe local reactions involving the upper airways and respiratory obstruction (asphyxia). In those, the respiratory tract may present massive edema, obstructive secretions, and total collapse or severe reduction in functional airway diameter.16 Massive envenomation from swarm stings can also cause death via non-allergic mechanisms.3 In the latter scenario, death may result from three major mechanisms: 1) direct venom toxicity, 2) intravascular hemolysis mediated by bee hemolytic factor, and 3) severe hypotension resulting from massive histamine release.16 Together, these mechanisms have a cumulative, cascading effect, resulting in multiorgan dysfunction syndrome (MODS) characterized by acute renal failure, respiratory distress. rhabdomyolysis, myoglobinemia, myocardial infarction, hepatic necrosis, disseminated intravascular coagulation (DIC), intravascular hemolysis, and hemorrhage.
Histopathologic findings in fatal bee envenomation are not specific. In cases of massive envenomation, acute renal tubular necrosis; fatty degeneration of the kidneys, liver, and myocardium; hyaline membrane disease; and splenic hemorrhage and infarction have been documented. Specifically, renal injury may result from the combination of hemoglobin/myoglobin noxious effect, direct toxicity and hypotension secondary to anaphylactic shock.6 Melittin and PLA2 may cause endothelial damage (angiopathy) leading to increased vascular permeability, thrombosis and infarction. The histopathological features observed in the presented kangaroo recapitulated these pathological findings documented in human and animal fatalities linked to bee massive envenomation. These lesions were the result of systemic microangiopathy, DIC and hemorrhage leading to and aggravated by shock with eventual death. To the best of our knowledge, this represents the first description of fatal bee envenomation in macropodids.
The diagnosis of bee massive envenomation is one of exclusion and should stem from a history of suspected or confirmed exposure to numerous bees matched with the onset of appropriate clinical signs and compatible pathologic findings. Furthermore, determination of tryptase levels (a mast cell-specific enzyme released upon mast cell degranulation) and venom-specific IgE are of postmortem diagnostic value in human forensics.5,10 Its usefulness has not been investigated in veterinary species; further research is warranted.
Contributing Institution:
Texas A&M Veterinary Medical Diagnostic Laboratory
483 Agronomy Rd
College Station, TX 77843
JPC diagnosis:
1. Heart, myocardium: Coagulative necrosis, multifocal to coalescing, with hemorrhage.
2. Liver, hepatocytes: Coagulative necrosis, subcapsular, massive, with diffuse, hepatocellular lipidosis.
3. Kidney: Acute tubular necrosis, multifocal to coalescing, with cellular and erythrocytic casts.
JPC comment:
The contributor provides a concise summary of this interesting case, and a great deal of the current knowledge regarding bee envenomation. While envenomation from the honeybee is always a concern for some individuals, the Africanized honeybee became a new threat to human and veterinary patients starting in the late 1980's. These bees (Apis mellifera scutellata) tend to be more territorial, faster to defend and attack, have slightly different composition of their venom, and have been called "killer bees" colloquially. After a colony of European honeybees is disturbed, it takes the bees approximately 2.9 minutes to "settle", but killer bees take 28.2 minutes to "settle". Killer bee venom contains higher concentrations of phospholipase A2 than European honeybee venom, the most allergenic component of the venom.13
While phospholipase A2 (also called Api m 1) is well characterized in bee venom, phospholipase A1 is more abundant in wasps and ants. It has been well characterized in fire ant (Solenopsis invicta) venom. The phospholipase A2 in bee venom belongs to a subgroup of small, secreted Ca2+-dependent phospholipase A2s with a highly conserved Ca2+ binding site. These molecules are dependent on millimolar concentrations of calcium for their catalytic activity. And while PLA2 is responsible for histamine release from leukocytes, it is also involved in LTC4 production and upregulation of CD63 in mast cells and basophils. Additionally, PLA2 induces production of IL-4, a critical component of the Th2 response and class switching to IgE in B-cells.11
As the contributor stated, intravascular hemolysis may be found clinically in animal affected by bee envenomation. Specifically, bee venom may cause hemolytic anemia, echinocytosis, spherocytosis, thrombocytopenia, hemoglobinemia, and hemoglobinuria. In the past, it had been assumed that in all cases, hemolytic anemia was immune-mediated, but recent cases have illustrated the cause of hemolysis to be destruction of erythrocytes by melittin forming large pores in the cellular membrane. PLA2 also causes echinocytosis, spherocytosis, and mitochondrial degeneration in platelets. However, in delayed reaction, some mechanisms of disease are still likely antibody mediated.8
References:
1. Abd El-Wahed AA, Khalifa SAM, Sheikh BY, et al. Bee venom composition: from Chemistry to biological activity. In: Atta-ur-Rahman, ed. Studies in Natural Products Chemistry. Oxford:Elsevier; 2017: 459-484.
2. Bonagura JD, Kirk RW. Management of bee and other Hymenoptera stings. In: Bonagura JD. Kirk RW, eds. Kirks Veterinary Therapy XII: Small Animal Practice. Philadelphia: WB Saunders; 1995.
3. Castells MC, Hornick JL, Akin C Anaphylaxis after hymenoptera sting: is it venom allergy, a clonal disorder, or both? J Allergy Clin Immunol Pract. 2015;3:350-355.
4. Danneels EL, Van Vaerenbergh M, Debyser G, Devreese B, de Graaf DC. Honeybee venomproteome profile of queens and winter bees as determined by a mass spectrometric approach. Toxins (Basel). 2015;7:4468-4483.
5. Elieh Ali Komi D, Shafaghat F, Zwiener RD. Immunology of Bee Venom. Clin Rev Allergy Immunol. 2018;54:386-396.
6. Hughes RL. A Fatal Case of Acute Renal Failure From Envenoming Syndrome After Massive Bee Attack: A Case Report and Literature Review. Am J Forensic Med Pathol. 2019;40:52-57.
7. Manoquerra AS. Hymenoptera stings. In: Ling L, Clark R, Erickson T, eds. Toxicology
secrets. Philadelphia: Hanley and Belfus; 2001.
8. Nair R, Riddle EA, Thrall MA. Hemolytic anemia, spherocytosis, and thrombocytopenia associated with honey bee envenomation in a dog. Veterinary Clinical Pathology. 2019;48(4):620-623.
9. Oliveira EC, Pedroso PM, Meirelles AE, et al. Pathological findings in dogs after multiple Africanized bee stings. Toxicon. 2007:49:1214-1218.
10. Payne V, Kam PC. Mast cell tryptase: a review of its physiology and clinical significance. Anaesthesia. 2004;59:695-703.
11. Perez-Riverol A, Lasa AM, Santos-Pinto JRA, Palma MS. Insect venom phospholipases A1 and A2: Roles in the envenoming process and allergy. Insect Biochemistry and Molecular Biology. 2019;105:10-24.
12. Pucca MB, Cerni FA, Oliveira IS, et al. Bee Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Front Immunol. 2019;10:2090.
13. Rahimian R, Shirazi FM, Schmidt JO, Klotz SA. Honeybee stings in the era of "killer bees": Anaphylaxis and toxic envenomation. The American Journal of Medicine. 2019; 133(5):621-626.
14. Rayamane AP Kumar MP, Kishor DG. Honey bee stings and anaphylaxis. J Forensic Med Sci Law. 2014;23:53-60.
15. Ribeiro PR, Bianchi MV, Henker LC, Gonzales F Pavarini SP. Acute renal failure in a horse following bee sting toxicity. Ciencia Rural. 2020;50:1-5.
16. Riches KJ, Gillis D, James RA. An autopsy approach to bee sting-related deaths. Pathology. 2002;34:257-262.
17. Schumacher MJ, Egen NB. Significance of Africanized bees for public health. A review. Arch Intern Med. 1995:155:2038-2043.
18. Staempfli HR, Wollenberg G, Jewell G, McCutcheon LJ. Acute fatal reaction to bee stings in a mare. Equine Veterinary Education. 1993;5:250-252.
19. Veado HC, Conceicao RS, Nogueira K, et al. Massive Africanized honeybee stings in two
hair sheep and a mare. Toxicon. 2020;177:35-40.
20. Vogelnest L, Portas T. Current Therapy in Medicine of Australian Mammals. Clayton South, Victoria, Australia: CSIRO Publishing, 2019.