Signalment:
4-year-old female German shorthaired pointer dog (
Canis familiaris).The dog was regularly used for hunting purposes. In January 2009 the owner noticed single coughing attacks after trailing a wounded animal. The next morning the dog showed apathy, inappetence and recurrent coughing attacks. Clinical observations revealed no other symptoms. Body temperature was normal. There were no abnormal auscultation findings of thorax and abdomen. Antibiotic therapy was initiated and the owner gave a cough syrup. In the evening the animal showed regurgitation of reddish-brown fluid, salivation and progressive deterioration with dyspnea. The dog was referred to a veterinary clinic immediately. There were no specific radiographic findings and bronchoscopy was inconclusive. The animal suddenly died 30 hours after the first attack of coughing. The clinicians suspected intoxication. Pseudorabies was discussed differentially but ruled out due to the lack of itch and other central nervous signs.
Gross Description:
Unfortunately, the owner did not permit a full necropsy. However, the clinician collected samples of stomach and intestine and removed the brain for histology and/or toxicology 48 hours post mortem
Histopathologic Description:
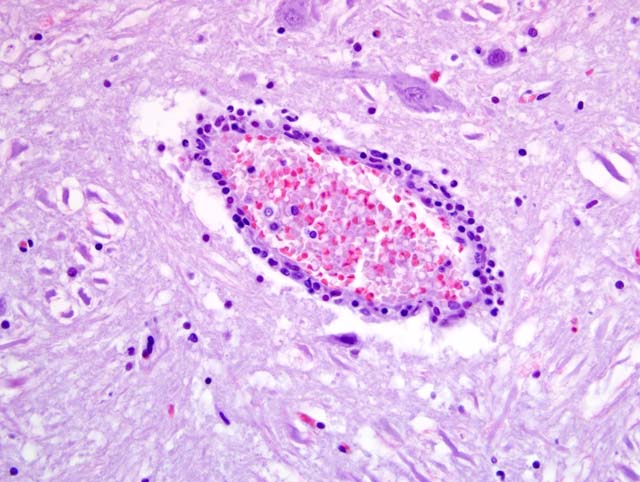
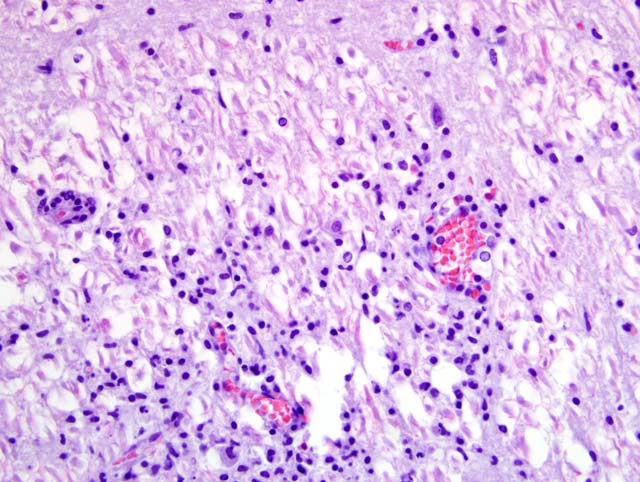
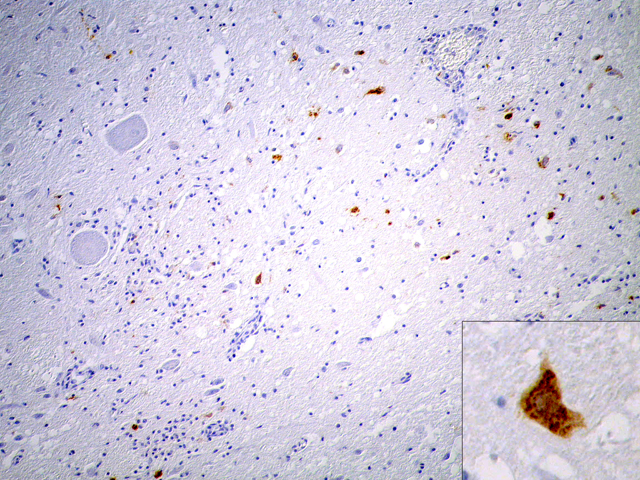
Due to the suboptimal brain sampling, slides contain an angular section of cerebellum and brainstem. In the brain stem there is a moderate perivascular infiltration and cuffing composed of mononuclear cells (lymphocytes and macrophages). Focally there are acute perivascular hemorrhages. Multifocally in the brainstem there are single degenerating neurons (dark neurons) and within the neuropil there are multifocal accumulations of activated microglial cells (rod cells, microgliosis) admixed with few infiltrating neutrophils. Single neurons and glial cells show intranuclear round eosinophilic inclusion bodies with chromatin margination. The cerebellum is unaltered. Immunohistochemically porcine herpesvirus 1 antigen was detected within neurons and glial cells using the PAP method. Initially, using fresh material from intestine and brain, herpesvirus DNA was not detectable with PCR or in cell culture. In paraffin embedded material, using the gB-region in a nested PCR, porcine herpesvirus DNA was detectable.
Morphologic Diagnosis:
Cerebellum and brain stem: encephalitis, nonsuppurative, multifocal, moderate with perivascular lymphohistiocytic cuffing, neuronal degeneration, microgliosis and perivascular hemorrhages; Aujeszky-�s disease, pseudorabies; German shorthaired pointer dog (
Canis familiaris).
Condition:
Pseudorabies
Contributor Comment:
Pseudorabies, also known as Aujeszky-�s disease, infectious bulbar paralysis or mad itch, is caused by suid herpesvirus 1, an alphaherpesvirus of the genus varicellovirus.(4) The disease was first described and its infectious cause identified by Alad+�-�r Aujeszky (1869-1933), a Hungarian veterinary pathologist, in 1902. An interesting historical essay was published in 2003 by K+�-�hler and K+�-�hler.(3) Farm animals in many countries in Europe are free of the disease. But the increasing population of wild pigs in some areas (especially in Germany) serves as a reservoir and there is a general risk of transmission to livestock.(8) Suid herpesvirus was the object of intensive virological research in the past and the virus serves as model for other alphaherpesvirus infections and for developing marker vaccines and DNA vaccines.(2,5,7,9) The typical clinical symptom in animals other than pigs is intense pruritus at the locus of inoculation and the outcome is lethal in nonnative hosts. Dogs can be infected by ingestion of raw meat from infected pigs (farm or wild pigs) or percutaneously (bites, trauma). The virus spreads centripetally after inoculation along nerves to the spinal ganglia and the central nervous system.(4) Symptoms in dogs are similar to rabies but the clinical course is much shorter. Gross lesions are limited to self trauma due to severe itching.(4) Histologically, nonsuppurative encephalitis with gliosis and ganglioneuritis (e.g. trigeminal or spinal ganglia) can be found. Typical herpesvirus inclusion bodies can be found in neurons and glial cells. Virus antigen can be detected immunohistochemically on paraffin-embedded formalin-fixed material.(4)
The clinical course in the present case was very rapid (only 30 hours) with early lethal outcome. Contact of the dog with a wild pig or blood and/or carcasses of hunted wild pigs can be presumed. Due to the coughing attacks as the predominant symptoms, inhalation as possible route of infection can be discussed. Unfortunately, lung material was not submitted for examination. Stomach and intestine were unaltered. Virus antigen could not be detected immunohistochemically within tissues other than the brain.
JPC Diagnosis:
Brainstem: Encephalitis, nonsuppurative, multifocal, mild to moderate, with gliosis.
Conference Comment:
Participants readily attributed the striking nonsuppurative encephalitis in this case to a viral etiology; however, nonsuppurative inflammation in the central nervous system (CNS) is not entirely specific for viral infections, with salmonellosis in pigs and neorickettsial infection (i.e. salmon poisoning) in dogs being notable examples of non-viral causes of nonsuppurative CNS inflammation.(4) There was variation with respect to the presence of intranuclear inclusion bodies and scattered neuronophagia, and most participants developed a differential diagnosis that included several viral etiologies. Many conference participants suspected canine morbillivirus as the etiology; however, this case lacks the characteristic morbilliviral intracytoplasmic and intranuclear viral inclusions, which are generally detectable in astrocytes, and occasionally ependymal cells and neurons, in dogs with canine distemper virus infection. In early canine distemper, lesions include demyelination, status spongiosus, astrocytic hypertrophy and hyperplasia, and variable syncytial cell formation, all of which are lacking in this case. In late-stage canine distemper, lesions include nonsuppurative perivascular cuffing, leptomeningitis, and choroiditis, with occasional gitter cells, and most participants who favored a diagnosis of distemper specifically favored old dog encephalitis associated with distemper.(4) Other viral etiologies considered by conference participants included rabies and the arboviruses.
Pseudorabies is an unusual alphaherpesvirus in that it is proficient in interspecies transmission. Conference participants briefly discussed two other closely-related alphaherpesviruses that share this capability: human herpesvirus-1 (HHV-1, herpes simplex virus) and cercopithecine herpesvirus-1 (herpes B virus, BV). Humans are the primary hosts of HHV-1, with a seroprevalence of approximately 80%. Infection in humans is generally mild, characterized by labial, oral, and ocular lesions, with encephalitis being a rare consequence of centripetal infection through the olfactory, optical, or trigeminal nerves. However, rabbits are exquisitely sensitive to HHV-1, where the virus is exclusively neurotropic and almost invariably fatal. Less susceptible species include rats, mice, and chinchillas.(6) An analogous situation exists with BV, which is endemic in macaques and a seroprevalence of 80-100% in most populations. Following initial replication in mucosal epithelial cells, the virus is transmitted by axonal transport to dorsal root ganglia, where it establishes latency characterized by a lack of viral replication and a limited pattern of transcription. Episodes of recrudescence are generally asymptomatic, or less frequently cause oral herpetic lesions. Humans, by contrast, are exquisitely sensitive to BV, with infection being almost uniformly fatal in the absence of early treatment with antiviral therapy.(1) Other alphaherpesviruses of veterinary importance were briefly reviewed in WSC 2009-2010, Conference 5, case II.Â
References:
1. Barry P, Marthas M, Lerche N, McChesney MB, Miller CJ: Virology research.Â
In: The Handbook of Experimental Animals: The Laboratory Primate, eds. Wolfe-Coote S, Bullock G, Petrusz P, pp. 572-573, Elsevier Academic Press, San Diego, CA, 2005
2. Ekstrand MI, Enquist LW, Pomeranz LE: The alpha-herpesviruses: molecular pathfinders in nervous system circuits. Trends Mol Med.Â
14:134-40, 2008
3. K+�-�hler M, K+�-�hler W: Zentralblatt f+�-+r Bakteriologie--100 years ago Alad+�-�r Aujeszky detects a 'new' disease--or: it was the cow and not the sow. Int J Med Microbiol.Â
292:423-427, 2003
4. Maxie MG, Youssef S: Nervous system.Â
In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 1, pp. 411, 416-417, 432-433. Elsevier Saunders, Philadelphia, PA, 2007
5. Mettenleiter TC: Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis--state of the art. Vet Res.Â
31:99-115, 2000
6. M+�-+ller K, Fuchs W, Heblinski N, Teifke JP, Brunnberg L, Gruber AD, Knopfleisch R: Encephalitis in a rabbit caused by human herpesvirus-1. J Am Vet Med Assoc
235:66-69, 2009
7. Pomeranz LE, Reynolds AE, Hengartner CJ: Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev.Â
69:462-500, 2005
8. Ruiz-Fons F, Vidal D, H+�-�fle U, Vicente J, Gort+�-�zar C: Aujeszky's disease virus infection patterns in European wild boar. Vet Microbiol.Â
120:241-50, 2007
9. Zuckermann FA: Aujeszky's disease virus: opportunities and challenges. Vet Res.Â
31:121-31, 2000