Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 4
18 September 2019
CASE IV: M18-02265 (JPC 4118633).
Signalment: 3 year 8 month-old, female Friesian, bovine (Bos taurus)
History: On a farm in the Hunter region of NSW Australia, 2/40 adult cows died suddenly. Three days prior to death, the herd had been moved onto a Kikuyu (Pennisetum clandestinum) paddock. It was also possible that the herd had not had access to water for 24h.
Gross Pathology: Necropsy was performed on 2 animals. Gross findings in the necropsied animals included severe dehydration and abundant liquid content of the rumen. Ruminal papillae tips had multifocal pale discoloration. Mucosa from reticulum and omasum presented with multifocal red areas. The cows were pregnant with fetuses aged at approximately 7-8 months gestation
Laboratory results: Results of serum biochemistry analysis on an animal from the same herd were consistent with the gross finding of severe dehydration (decreased renal perfusion resulting in mild elevations in Urea/Creatinine/Phosphate and Protein/Albumin/Globulin) and muscle damage possibly related to being recumbent prior to death (mild elevations in creatinine and AST). Serum D-lactate levels were at the top of the normal range
|
|
|
|
Reference Range |
|
GGT |
27 |
|
0-35 U/L |
|
GLDH |
20 |
|
0-30 U/L |
|
AST |
181 |
H |
0-120 U/L |
|
BIL |
3.0 |
|
0.0-24.0 umol/L |
|
CK |
6752 |
H |
0-300 U/L |
|
UREA |
17.5 |
H |
2.1-10.7 mmol/L |
|
CREAT |
492 |
H |
0-186 umol/L |
|
PHOS |
3.28 |
H |
0.80-2.80 mmol/L |
|
URE/CREA |
0.04 |
|
0.00-0.07 |
|
PROTEIN |
128.5 |
H |
60.0-85.0 g/L |
|
ALBUMIN |
49.6 |
H |
25.0-38.0 g/L |
|
GLOB |
78.9 |
H |
30-45.0 g/L |
|
ALB/GLOB |
0.6 |
L |
0.7-1.1 |
|
BHB |
0.80 |
|
0.00-0.80 mmol/L |
|
CA |
2.62 |
|
2.00-2.75 mmol/L |
|
MG |
2.02 |
H |
0.74-1.44 mmol/L |
|
D-LACT |
0.5 |
|
0.0-0.5 mmol/L |
A Total Alfatoxins qualitative strip test on a sample of the kikuyu was negative (<4ppb cut off)
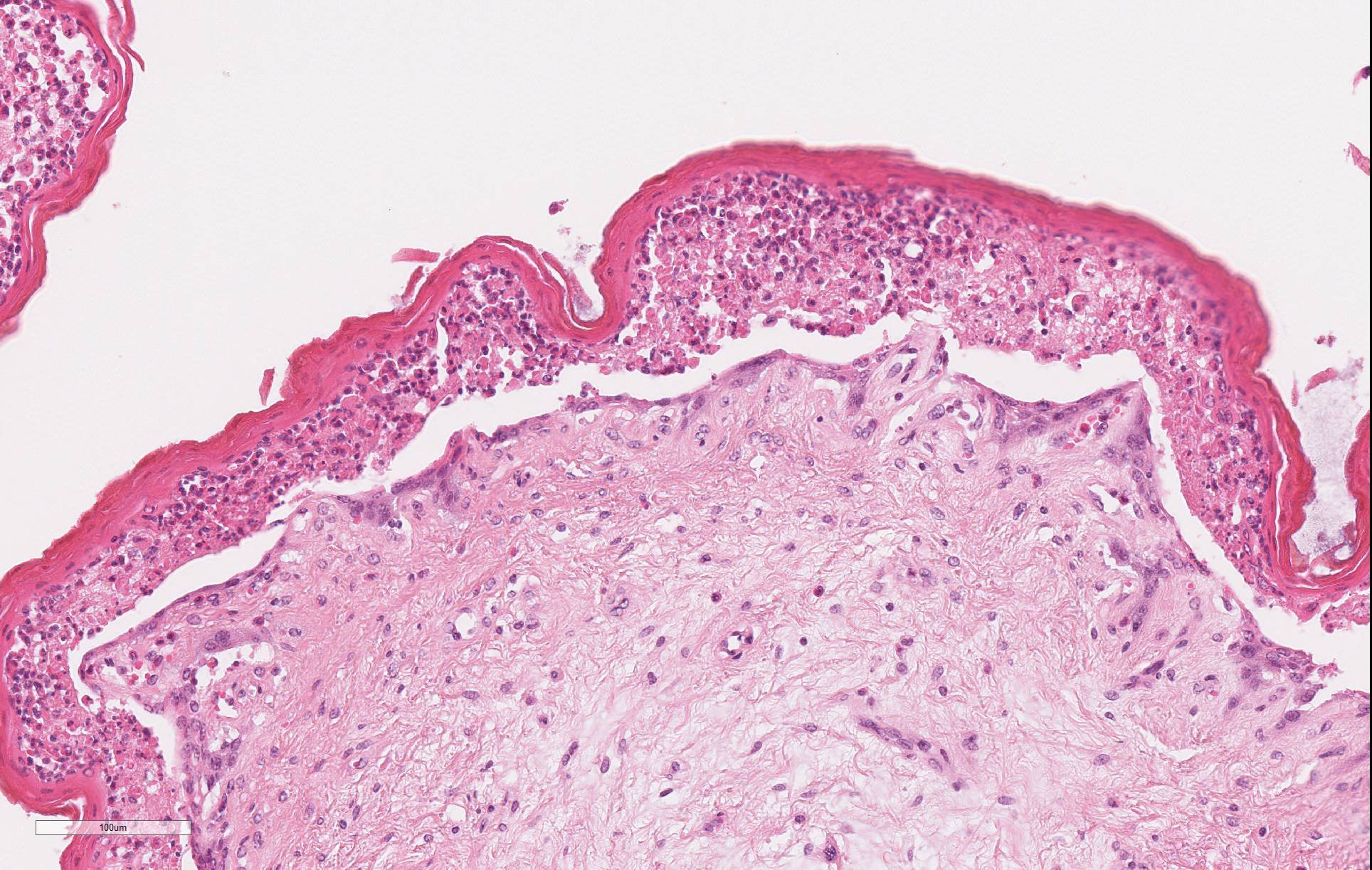
Microscopic Description: Omasum: There is widespread disruption of epithelial tissue architecture with multifocal to coalescing aggregates of degenerate neutrophils, epithelial cells with eosinophilic cytoplasm and shrunken, pyknotic or karrhorhectic nuclei admixed with cellular debris (micropustules). There is diffuse separation of omasal mucosa from underlying tissue (interpreted to be partially due to artifact). The omasal lumen is filled with fragments of refractile plant material mixed with myriad of small basophilic cocci. The submucosa is diffusely, mild to moderate expanded with clear areas (edema), with multifocal mild to moderate coalescing infiltrates of neutrophils, lymphocytes and plasma cells with occasional macrophages.
Reticulum: There is multifocal to coalescing disruption of epithelial tissue architecture with multifocal to coalescing aggregates of degenerate neutrophils, epithelial cells with eosinophilic cytoplasm and shrunken, pyknotic or karrhorhectic nuclei (interpreted as necrotic epithelial cells) (micropustules) admixed with clear spaces (edema) and cellular debris. Subepidermal vessels are diffusely congested and interstitial fibroblasts and myofibers are multifocal separated out by increased amounts of clear space.
Contributor Morphologic Diagnosis: Omasum: Omasitis, necrosuppurative, diffuse, moderate to severe with intracorneal pustules, Friesian, bovine (Bos taurus)
Reticulum: Reticulitis, necrosuppurative, multifocal to coalescing, moderate with intracorneal pustules, Friesian, bovine (Bos taurus)
Contributor Comment: Kikuyu grass (Pennisetum clandestinum) is a perennial tropical pasture species commonly found on coastal regions of Australia growing between spring and autumn.2 Poisoning occurs after ingestion of kikuyu after a period of rapid growth in autumn following a period of summer drought. From a collation of outbreaks across 40 farms, between 13.6% and 64.2% cattle were affected of which between 16.7% and 95.6% died.2 In an outbreak, signs of toxicity typically present on average 3 days (1-8 day range) post first grazing of kikuyu, with some cases progressing quickly to death over a subsequent week long period.2 Clinical signs consist of drooling, dehydration, abdominal pain, sham drinking, depressed mentation, incoordination and recumbency. Other signs may include distended abdomen, rumen stasis, elevated temperature and both cardiac and respiratory distress.2,3 A history of grazing kikuyu and a post mortem finding of a distended rumen containing a sloppy mix of pasture and fluid are highly suggestive of kikuyu poisoning. Additional supportive evidence from a post mortem includes hyperaemic mucosa of the forestomaches and abomasum, empty small intestine, dry contents in the large intestine and cardiac haemorrhages.2 The most consistently reported histopathological lesion is segmental necrotising inflammation within the epithelium of the forestomach mucosa,2-4 most frequently the omasum.2 Cases of longer duration of exposure show mucosal inflammation regresses and epithelial repair occurring.2
There are multiple theories towards the toxin production that causes kikuyu poisoning. While produced seasonally, it is unknown if it is produced spontaneously by the plant, in response to a stimulus such as a pathogen or by a pathogen itself. Despite no reported cases of nitrate poisoning from kikuyu, one suggestion is the accumulation of nitrogenous compounds may be associated with toxin production.2 Another is the association of armyworms and kikuyu in some but not all cases1. Oral ingestion of fungi cultures and mycotoxins has produced similar kikuyu-like poisoning conditions in cattle, however isolation of these fungi and/or mycotoxins from kikuyu plants and soil associated with poisonings is not consistent.1,2,6
Other diseases with a similar presentation to kikuyu poisoning include metabolic disease such as hypocalcaemia and hypomagnesemia, infectious diseases such as listeriosis and Histophilus somni meningoencephalitis, or other poisonings such as lead, salt or nitrate poisoning. Ruminal acidosis causes similar histological lesions, but is usually restricted to the rumen.
Contributing Institution: Elizabeth Macarthur Agricultural Institute, NSW, DPI
JPC Diagnosis: 1. Omasum:
Omasitis, necrotizing, multifocal to coalescing, with numerous intracorneal
pustules.
2. Reticulum: Reticulitis, necrotizing, multifocal to coalescing, with
numerous intracorneal pustules.
JPC Comment: Kikuyu grass (Pennisetum clandestinum) is a drought-tolerant grass native to the region of Kenya that is home to the Kikuyu people. It has been adapted to be a lawn grass in dry regions of New Zealand, South Africa, and the Southwestern US (California). It has become popular on golf courses due to its dense nature and propensity to create challenging rough. It has been termed an invasive species in several countries due to its ability to overgrow other plant life, preventing other plants from sprouting and producing herbicidal toxins that compete with other plants.5
Toxicity is sporadic, with many animals grazing it with no ill effects. Outbreaks are brief, geographically restricted, and lethal. A number of strains (with such names as "Whittet", "Crofts", and "Noonan") of kikuyu grass have been developed but do not appear to differ significantly in potential toxicity.2
The contributor mentions the "sloppy mix" of pasture and fluid in the rumen. This appears to be the result of a number of contributing factors, to include excess salivary production, ruminal stasis and a net outflow of fluid from the ruminal mucosa into the ruminal lumen. Animals will excessive amounts of ruminal fluid may "sham drink" (placing the head in or near water or playing in a water source without actual drinking) and die of dehydration due to third spacing of water into the rumen.2
The microscopic lesion demonstrated in this case is consistent with that described in cases of kikuyu poisoning - necrosis of the superficial layers of the ruminal, reticular, and omasal mucosa (stratum lucidum, granulosum, and spinosum) with profound infiltration by neutrophils which lifts it off of the underlying stratum basale. Degenerative changes in the myocardium and kidney have been infrequently reported in association with this condition.2
The identity of the toxin in kikuyu grass is yet to be elucidated and is the subject of many theories regarding its identity and seasonality. The forestomach lesion is reminiscent of that seen with ingestion of Baccharis sp. plants by ruminants (WSC Conference 2012-2013, Conference 4, Case 2), which is the result of accumulation of fungus-produced trichothecene toxins. A kikuyu-lke condition has been reproduced in ruminants via feeding of fungal cultures from two pasture fungi, Myrothecium sp. and Phoma sp. which produce roridin and verrucarin toxins, respectively. Nitrates have often been incriminated in outbreaks of kikuyu grass, which accumulated nitrates during their reproductive period within their stems. Leaf damage by various insects have been theorized to increased the consumption of kikuyu stems in some outbreaks, or overgrazing may increase stem consumption. In most reported cases of kikuyu poisoning, nitrate levels were within normal limits in affected animals.2
References:
1. Botha C, Truter M, Jacobs A. Fusarium species isolated from Pennisetum clandestinum collected during outbreaks of kikuyu poisoning in cattle in South Africa. Onderstepoort J Vet Res. 1983;81(1):1-9. doi:10.4102/ojvr.v81i1.803.
2. Bourke C. A review of kikuyu grass (Pennisetum clandestinum) poisoning in cattle. Aust Vet J. 2007;85(7):261-267. doi:10.1111/j.1751-0813.2007.00168.x.
3. Gwynn R, Gabbedy B, Hophinkson W, Kay B, Wood Pm. Kikuyu Poisoning of Cattle in Western Australia. Aust Vet J. 1974;50(8):369-370. doi:10.1111/j.1751-0813.1974.tb14116.x.
4. Martinovich D, Smith B. Kikuyu Poisoning of Cattle 1. Clinical and Patholgical Findings. N Z Vet J. 1973;21(4):55-63. doi:10.1080/00480169.1973.34078.
5. "Pennisetum clandestinum (General impact)" Global Invasive Species Database, Invasive Species Specialist Group. (http: //www.issg.org/database/species/impact_info.asp?si=183&fr=1&sts=&lang-EN.
6. Ryley M, Bourke C, Liew E, Summerell B. Is Fusarium torulosum the causal agent of kikuyu poisoning in Australia? In: Australasian Plant Disease Notes.Vol 2. Australasian Plant Pathology Society; 2007:133-135. doi:10.1021/jf991022b.