Signalment:
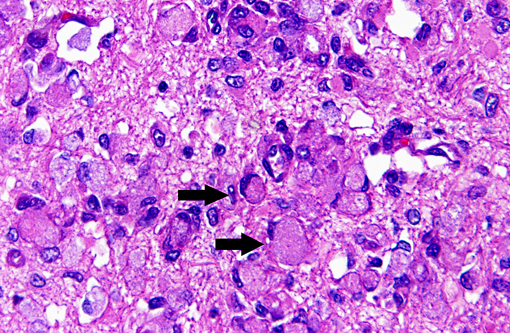
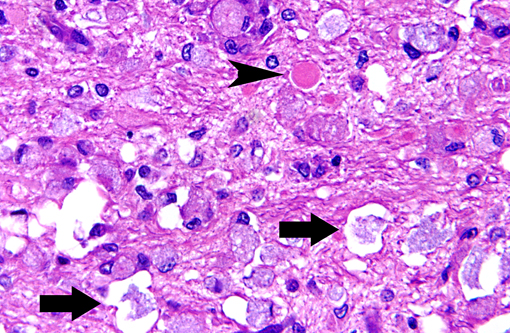
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Globoid cell leukodystrophy, also known as galactocerebrosidosis, is an autosomal recessive disease caused by deficiency in the activity of lysosomal galactocerebrosidase (GALC)(1,2). Globoid cell leukodystrophy is part of the sphingolipidosis group of lysosomal storage diseases. The enzymatic deficit blocks the catabolism of galactocerebroside (galactosylceramide), a major component of myelin, and therefore the metabolic function of oligodendrocytes and Schwann cells is affected. The enzyme is also involved in the breakdown of other metabolites, including galactosylsphingosine (psychosine), normally synthesized by oligodendrocytes. This latter substance is cytotoxic to oligodendrocytes. Psychosine accumulation leads to oligodendrocyte degeneration and death, ceasing of myelination and degeneration of formed myelin(1). Psychosine was sufficient to induce axonal defects and cell death in cultures of acutely isolated neurons in twitcher mice, a murine model of globoid cell leukodystrophy. Axonopathy in young twitcher mice occurred in the absence of demyelination and of neuronal apoptosis(3).Â
Macrophages accumulate to ingest the degenerating myelin, but are unable to degrade galactocerebroside, and give rise to the distinctive, swollen, PAS positive globoid cells. The cells are centered around blood vessels in the white matter, leptomeninges, and endoneurium of peripheral nerves. At the end stage of the disease there is diffuse demyelination, axonal loss, and dense astrogliosis(1). On electron microscopy, cytoplasmic contents include myelin membranes in various states of degeneration, and aggregates of straight or arched tubules of galactosylceramide bound by a membrane.
Neurological signs in dogs typically appear in young animals (often between 3 and 6 months of age). Clinical signs associated with this disease are variable and may reflect the multifocal distribution of lesions(2). Clinical signs include ataxia, hypermetria, tremors, and proprioceptive deficits. Progression leads to blindness, anorexia, muscular atrophy, and paraplegia. Death may occur before one year of age. The disease has been reported in a variety of canine breeds including: Cairn terrier, West Highland white terrier, miniature poodle, bluetick hound, basset hound, beagle(1), Pomeranian, Australian kelpie(4), Irish setter(5) and others. In addition to dogs, the disease is reported in domestic cats, polled Dorset sheep, rhesus macaques, and humans. There is a study model for the disease based on the twitcher mouse(3). Up to date there are 33 distinct GALC mutant alleles reported in humans(6).
JPC Diagnosis:
Conference Comment:
The conference moderator noted that gitter cells can resemble globoid cells on H&E stained sections and that both will have PAS positive cytoplasmic material; however, globoid cells are only found in the white matter and have a distinctly eccentric nucleus. Additionally, the presence of reactive endothelium can help to differentiate true pathologic findings from artifact or autolysis, and they may be the most noticeable pathologic change on subgross examination to indicate the presence of a microscopic lesion.
There was marked variation of anatomic location among the slides of conference participants. This should not detract from the diagnosis, but may reflect a difference in the histologic description compared to contributor slides.
References:
2. Braund K. G. Storage Disorders. In: Braund's Clinical Neurology in Small Animals: Localization, Diagnosis and Treatment, Vite C.H. and K.G. Braund (Eds.) International Veterinary Information Service, Ithaca NY (www.ivis.org), A3219.0203, 2003
3. Castelvetri L.C , Givogri M. I, Zhu H, Smith B, Lopez-Rosas A, Qiu X, Van Breemen R, Bongarzone E. R. Axonopathy is a compounding factor in the pathogenesis of Krabbe disease. Acta NeuropatholM/i> [Epub ahead of print], 2011
4. Fletcher J. L, Williamson P, Horan D, Taylor R. M. Clinical signs and neuropathologic abnormalities in working Australian Kelpies with globoid cell leukodystrophy (Krabbe disease). JAVMA, Vol 237, No. 6, 2010
5. McGraw R. A, Carmichael K. P. Molecular basis of globoid cell leukodystrophy in Irish setters. Vet J 171(2):370-2, 2006
6. Tappino B, Biancheri R, Mort M, Regis S, Corsolini F, Rossi A, Stroppiano M, Lualdi S, Fiumara A, Bembi B, Di Rocco M, Cooper D. N, Filocamo M. Identification and characterization of 15 novel GALC gene mutations causing Krabbe disease. Hum Mutat 12: E1894-914, 2010