Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 19
19 February, 2020
Dr.
Molly Church, DVM, PhD, DACVP
Assistant Professor
Pathobiology
University of Pennsylvania School of Veterinary Medicine
Philadelphia, PA
WSC 1920 Conf 19 Case 4 (JPC 4033564).
Signalment: 16-year-old, female spayed Maine coon cat (Felis catus)
History: The cat originally presented for an acute episode of collapse, left hemiparesis, and increased respiratory rate and respiratory effort. Neurological examination revealed anisocoria, a left head turn, and ambulatory left hemiparesis with proprioceptive deficits in the left fore and hind limbs. The neuroanatomical localization was multifocal with suspicion for C1-C5 myelopathy. Hematology and serum biochemistry profile were unremarkable, with the exception of a moderately elevated creatine kinase (1024 IU/L, RI: 64-440 IU/L). Thoracic radiographs revealed a patchy interstitial lung pattern presumed secondary to parenchymal disease. MR imaging of the brain and cervical spinal cord revealed an ill-defined hyperintensity in the left ventrolateral portion of the spinal cord at the cranial aspect of the C3. No intracranial abnormalities were detected, and an incidental unilateral otitis media was observed. Cisternal CSF analysis revealed moderately increased protein (184 mg/dL) and a moderately increased nucleated cell count with a mixed cell pleocytosis (150 nucleated cells/uL, 52% small mononuclear cells/mature lymphocytes, 27% nondegenerative neutrophils and 21% large monocytoid mononuclear cells with scattered erythrocytes and rare erythrophagia). A vascular event was the most likely differential, but infectious etiologies could not be completely ruled-out. Titers for Cryptococcus and Toxoplasma were declined by the owner. The following day the cat?s neurological examination and ability to ambulate had already improved. The cat was discharged with antibiotics and instructions to recheck with the neurology service and consult with the cardiology department. Serial neurological examinations revealed mild persistent paraparesis.
Eighteen months later the cat represented in congestive heart failure with a history of chronic progressive paraparesis. The owner elected euthanasia.
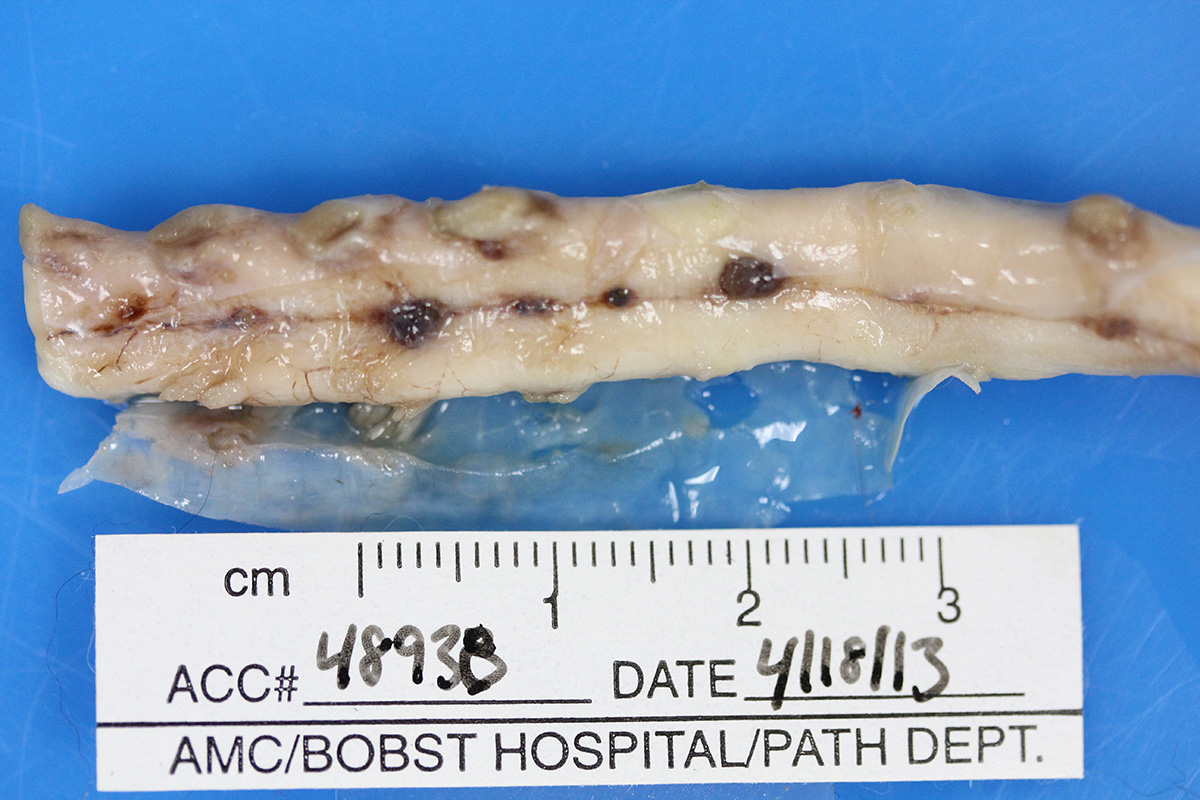
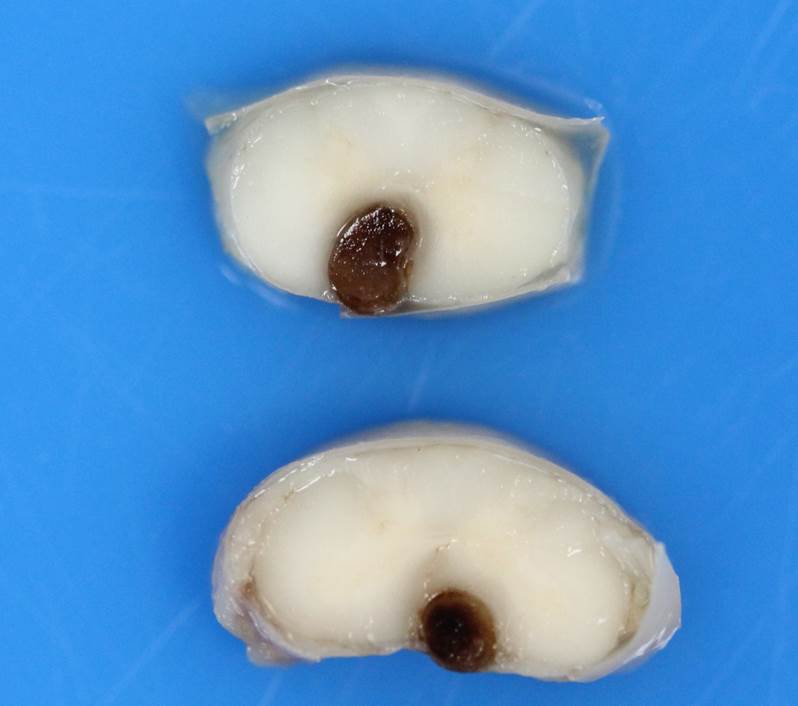
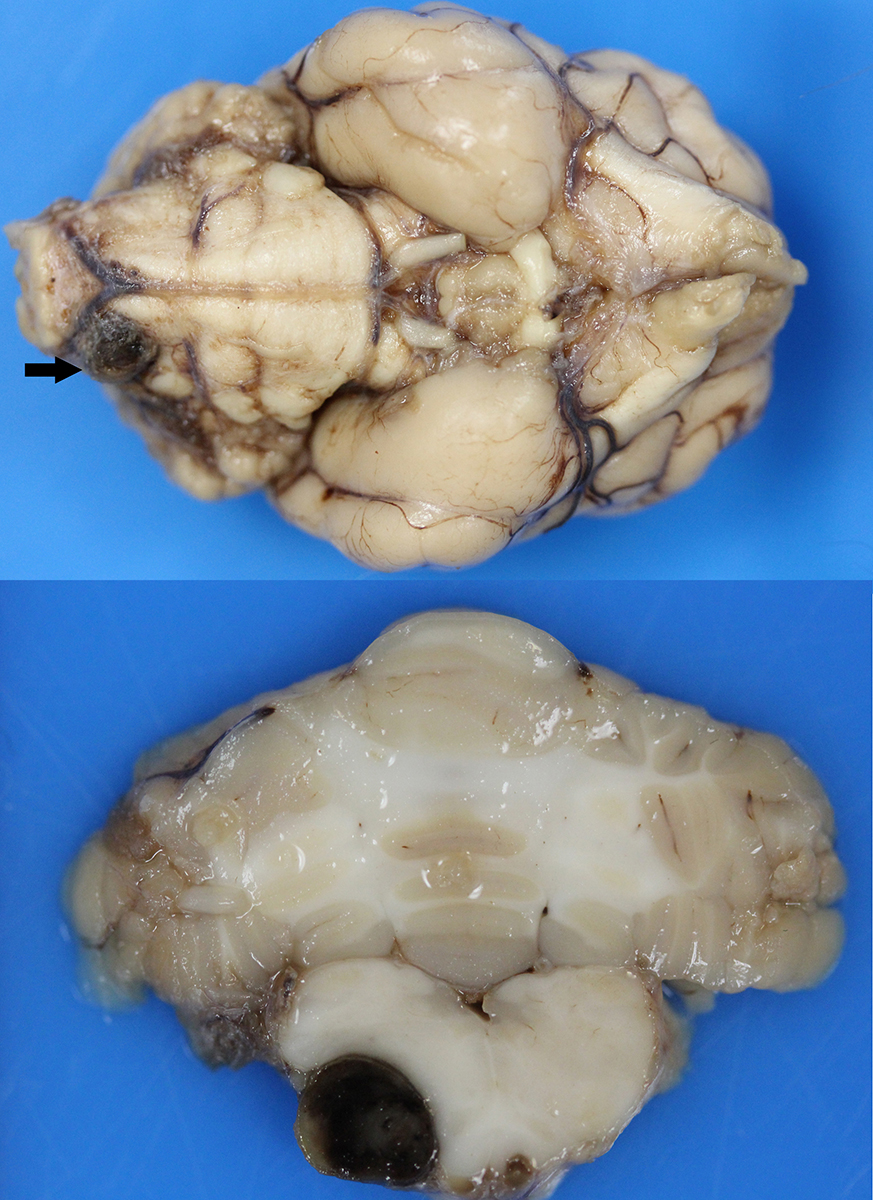
Gross Pathology: Macroscopic examination of the spinal cord revealed four large, multifocal, dark red, raised nodules (presumed telangiectasia) at the ventral aspect of the mid body of C3-C4 and C4-C5 (Figure 1A), involving the cervical intumescence. These foci ranged from 3 x 2 mm to 1 mm in diameter. An additional, similar, small, dark red focus at ventral midline of the distal thoracic spinal cord measured approximately 1 mm in diameter, and three dark red, slightly raised, nodules were present on the ventral aspect of the lumbar spinal cord at L3 and L4, the largest measuring 5 x 2.5 mm (Figure 1B). When the nodules were sectioned, they were found to be homogenously dark red, and extended into the spinal cord parenchyma, at least 2.5 mm into the cervical segments (Figure 2), and 3 mm into the lumbar segments. In the brain, a large, well-demarcated, dark red, homogeneous, firm, round mass at the ventral lateral aspect of the medulla measured 6 x 5 x 7 mm and was adjacent to a branch of the basilar artery (arrow, Figure 3). When sectioned, the mass was well-demarcated, homogeneously dark red, and compressed the adjacent medullary parenchyma (Figure 3). A nodule in the left thyroid gland was observed, and the heart was moderately enlarged with dilation of the left atrium and auricle. There was no evidence of thromboembolism in the caudal aorta or iliac arteries.
Laboratory results: Serum biochemistry profile revealed a mildly elevated T4 concentration (5.0 ug/dL, (RI: 0.8-4.7 ug/dL) and mild azotemia (BUN 51 mg/dL, RI: 14-36 mg/dL; creatinine 2.0 mg/dL, RI: 0.6-2.4 mg/dL).
Microscopic Description:
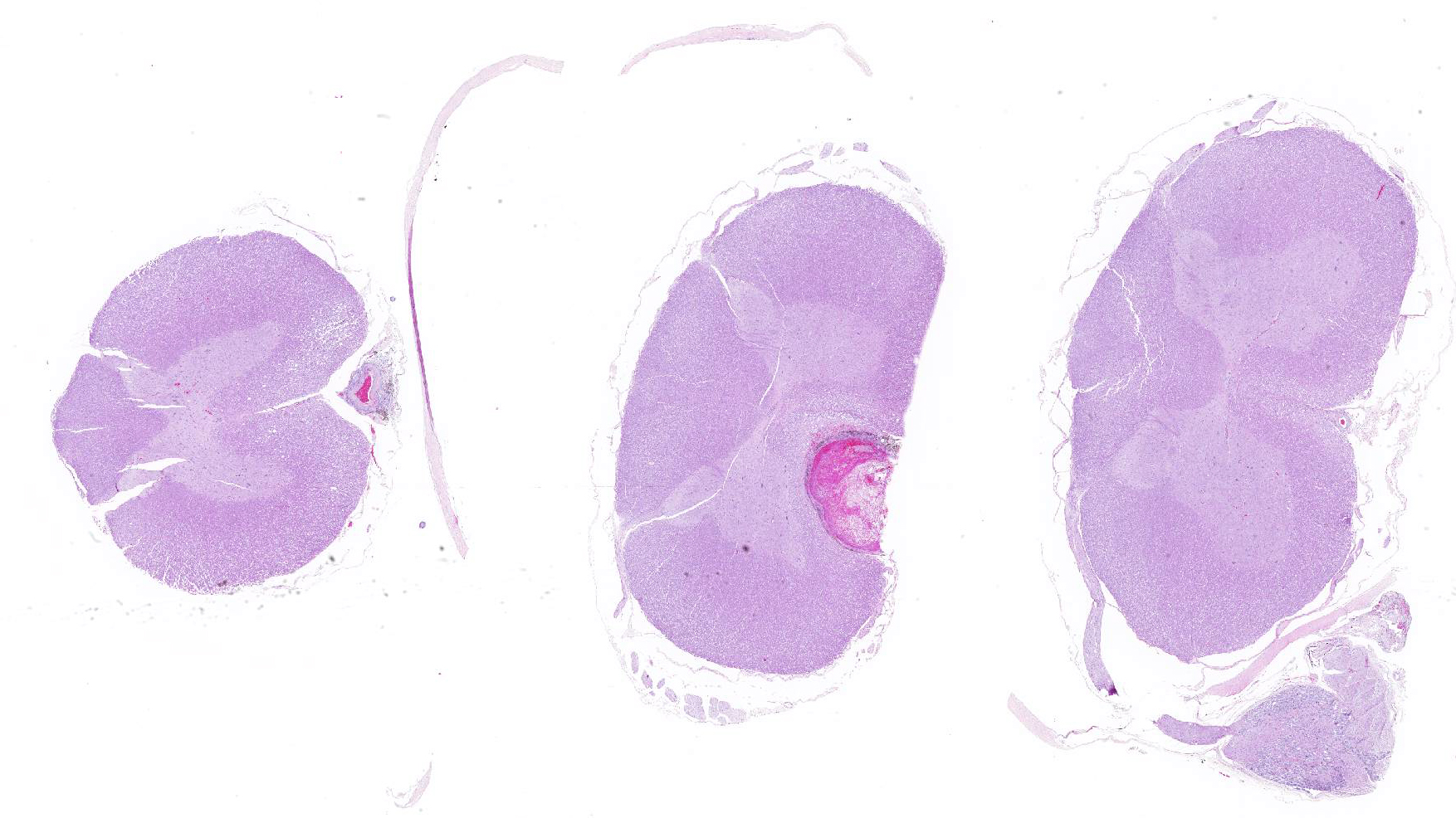
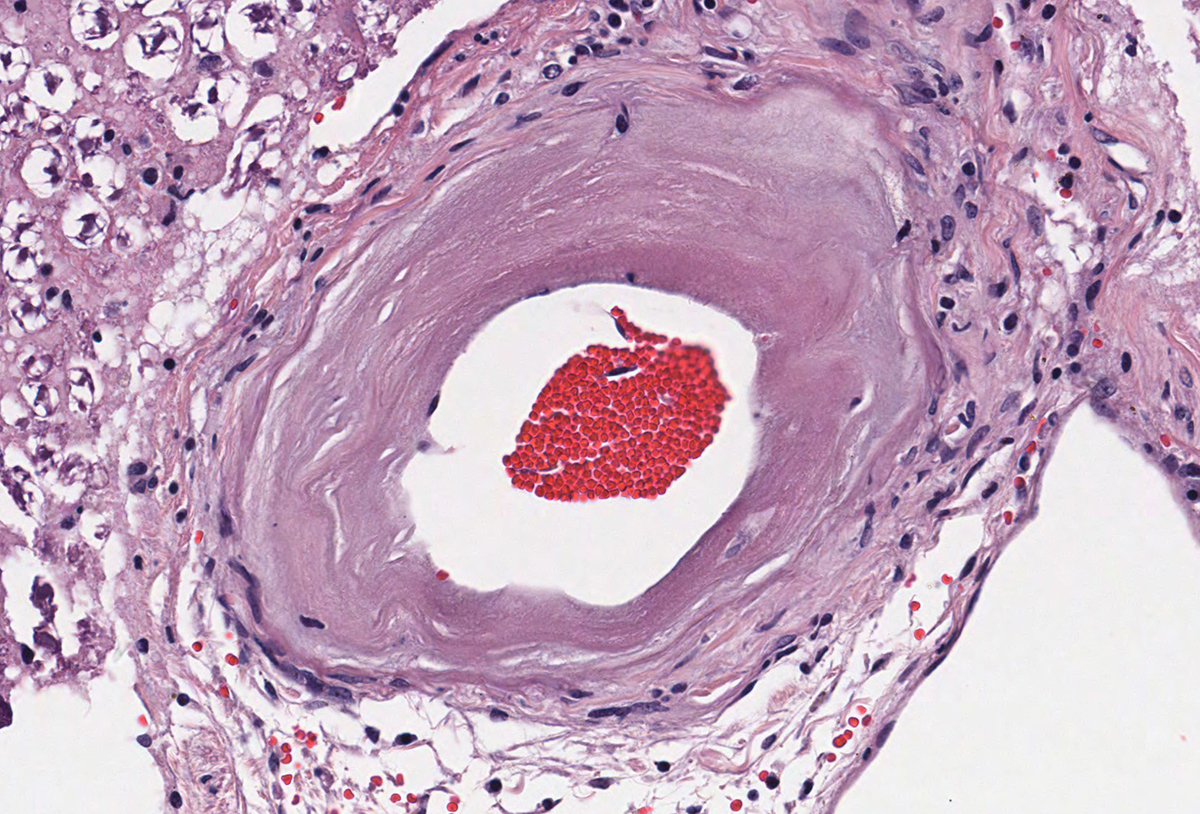
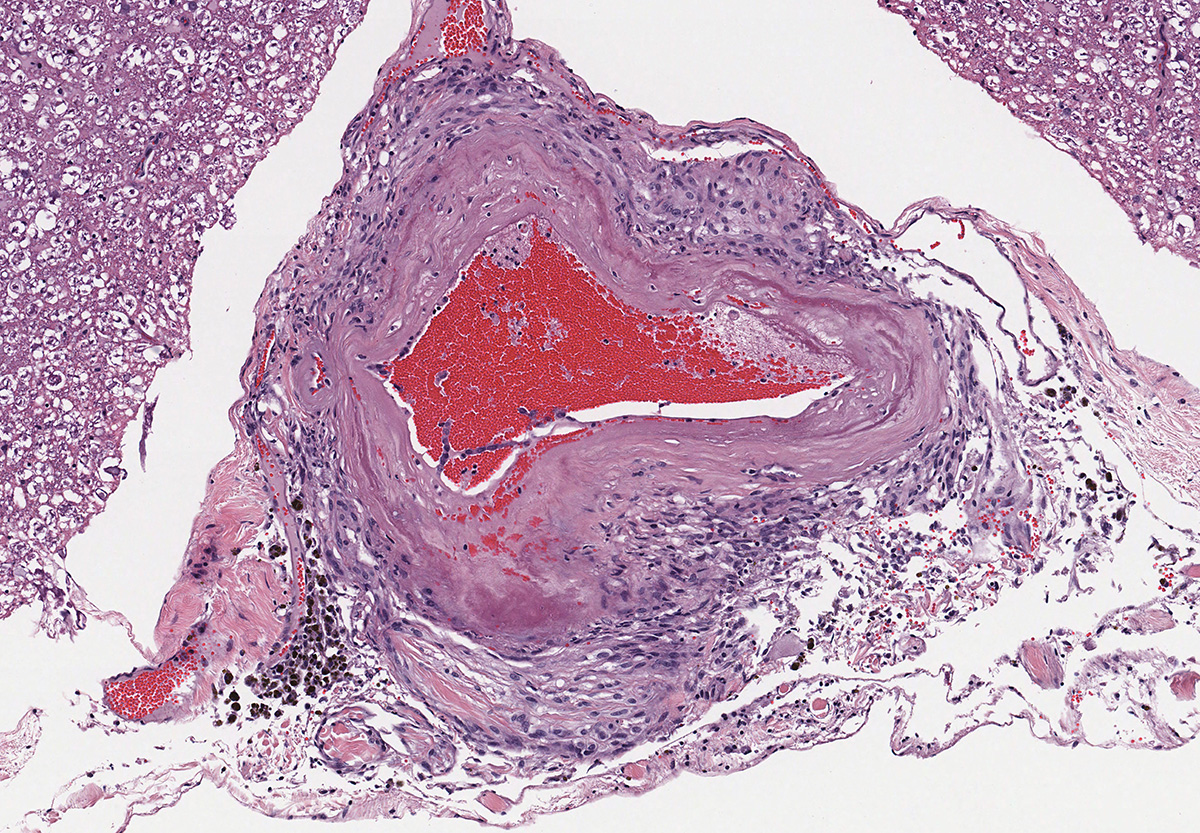
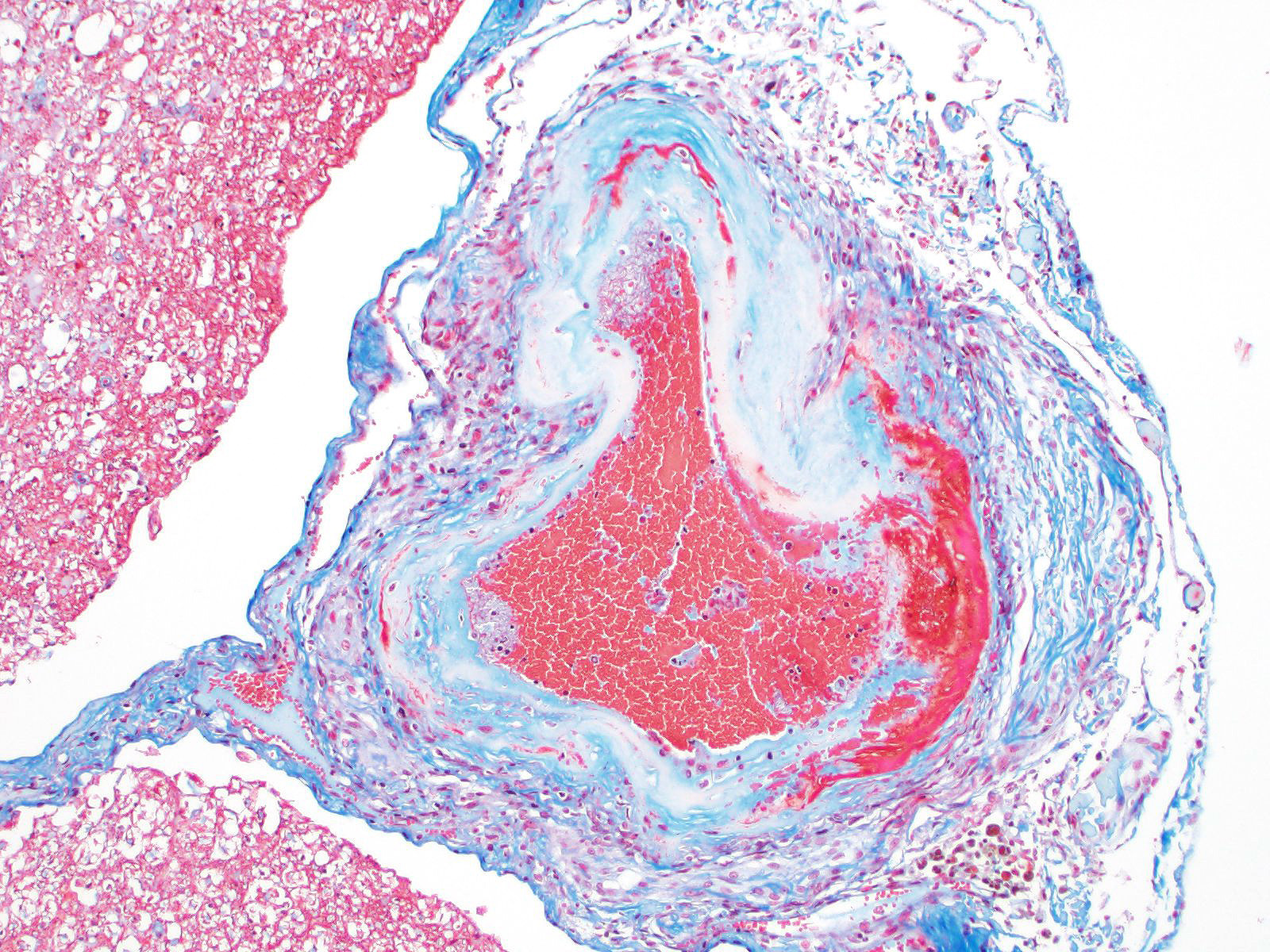
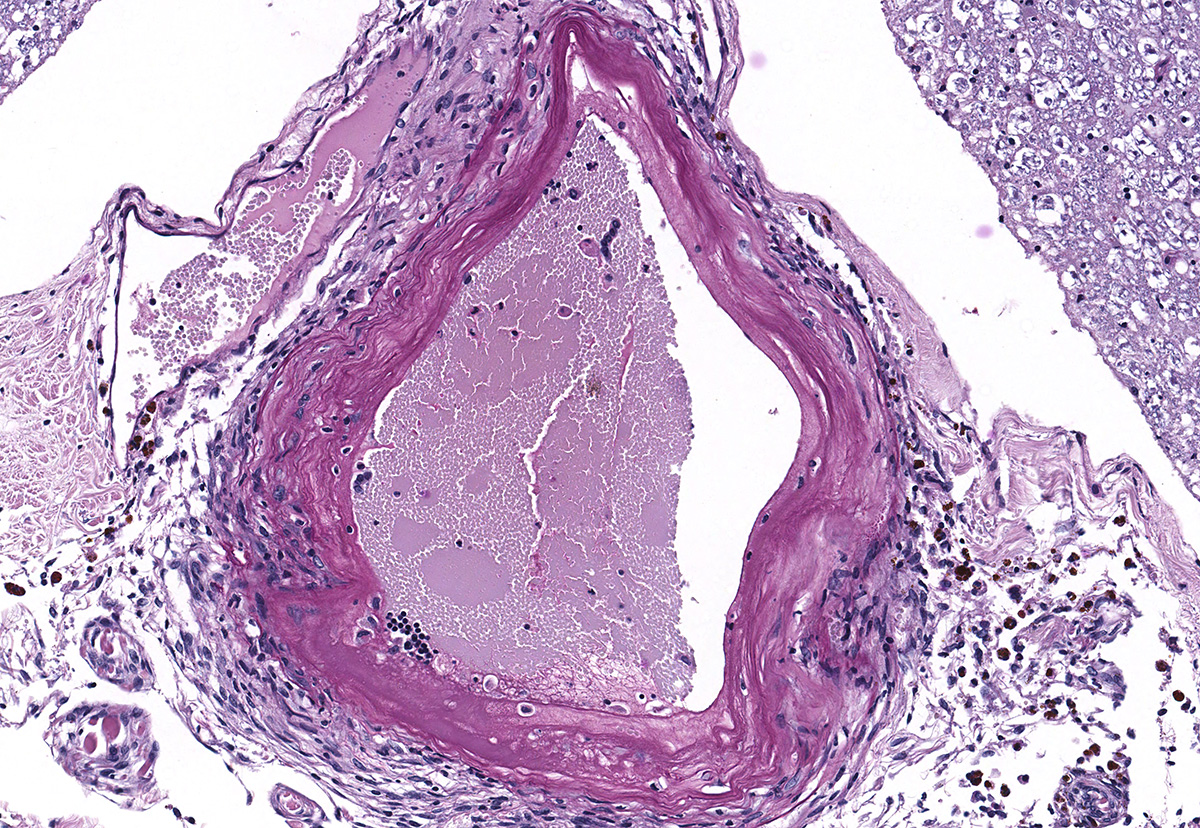
Microscopic evaluation of the spinal cord revealed severe aneurysmal dilation of the ventral spinal artery at C3-4, C4-5 (Figure 4), L3 and L4. The tunica intima and media of the dilated vessels were thickened by deposition of hyaline, acellular eosinophilic material (hyalinosis, Figure 5) and occasionally, increased populations of spindle cells. In regions of most severe arterial dilation, the arterial wall was attenuated. Luminal thrombi were present within the affected arteries, with foci of re-canalization. Intramural arterial and periarterial hemorrhage was observed, with periarterial hemosiderin- and hematoidin- containing macrophages (Figure 6), sometimes intermixed with lymphocytes and plasma cells. These severely dilated vessels compressed the adjacent parenchyma. The ventral funiculi of the cervical and lumbar spinal cord exhibited variable amounts of white matter myelin vacuolation, with swollen, eosinophilic axons (spheroids), rare myelomacrophages, increased populations of glial cells, including microglia with rod morphology and reactive astrocytes, few Gitter cells, hemorrhage, and accumulation of eosinophilic material (possible edema). Within the ventral grey matter of the lumbar section, there was mild hemorrhage. Few corpora amylacea and Rosenthal fibers were present, most notably in the section of lumbar spinal cord. Occasional aggregates of eosinophilic material were observed in spinal nerves. A focus of hemorrhage intermixed with few hemosiderin containing macrophages was adhered to the dura in a section of cervical spinal cord. In regions of vascular hyalinosis, Congo Red stains were negative for amyloid, and hyaline material stained magenta with Periodic acid Schiff (PAS) stains (Figure 7).
Histologic evaluation of the medullary mass in the brain (not included) revealed a severely dilated, thrombosed artery, adjacent to a lateral branch of the basilar artery (presumed to represent a communicating branch), with compression of the surrounding brain parenchyma and midline shift at the level of the olivary nucleus and deep arcuate fibers. Vessels were attenuated or the tunica intima and media were thickened by deposition of hyaline, acellular eosinophilic material (hyalinosis) and less frequently, spindle cell populations, with thrombosis, mural hemorrhage, periarteriolar hemosiderin-containing macrophages and lymphocytes. Congo red stains were negative for amyloid in regions of arterial hyalinosis. The regional white matter exhibited myelin vacuolation, spheroid formation and necrosis, characterized by white matter rarefaction and Gitter cell infiltration, with rare neuronal chromatolysis and necrosis.
Contributor?s Morphologic Diagnosis:
Spinal cord (cervical and lumbar segments): Severe, focal to multifocal ventral spinal hyaline arteriopathy (arteriolosclerosis) with severe dilation, thrombosis, intramural and periarteriolar hemorrhage and hemosiderosis
Spinal cord (cervical and lumbar segments): Severe, ventromedial white matter vacuolation with spheroid formation, gliosis, edema and hemorrhage
Contributor?s Comment: The ventral spinal artery was severely dilated, thrombosed, and thickened by deposition of hyaline material, resulting in grossly visible lesions. Damage to the spinal cord was interpreted to be multifactorial, caused by both ischemic and compressive damage. Arteriosclerosis literally translates to ?hardening of the arteries? and in veterinary medicine, encompasses arteriolosclerosis and atherosclerosis. In humans, arteriosclerosis includes the two aforementioned categories as well as Monckeberg medial calcific sclerosis.3 These lesions all result in stiffening and thickening of the arterial wall.3
Arteriolosclerosis is defined as a lesion of arterioles, which are small arterial vessels with 1 or 2 layers of smooth muscle cells.3 Arteriolosclerosis includes hyaline and hyperplastic lesions.1,,3,8 In humans, arteriolar hyalinosis occurs with benign hypertension and is associated with impaired autoregulation, hypothesized to be caused by hemodynamic injury with leakage of plasma components into the vessel wall.1,9 This lesion can also be seen with aging, diabetes mellitus, and focal segmental glomerulosclerosis (FSGS).9 Hyperplastic arteriolosclerosis in people is more commonly linked to malignant hypertension, the histologic changes of which includes thickening of the arteriolar wall by concentric layers of hyperplastic smooth muscle cells. Recommendations have been made to term the hyaline subtype as intimal hyalinosis and hyperplastic arteriolosclerosis as fibromuscular intimal thickening.3
Hypertension has been linked to arteriolosclerosis in cats, reported in association with chronic renal disease, hyperthyroidism, primary hyperaldosteronism, and chronic anemia.4,6,7 Between 65 and 100% of cats with systemic hypertension and concurrent hypertensive ocular lesions have evidence of reduced renal function.4 However, this relationship is complex, and it cannot always be determined whether systemic hypertension is a cause or consequence of renal damage.4 Systemic hypertension may be less prevalent in the hyperthyroid feline population than previously thought (reported ranges from 9-23%), and approximately 20% will develop hypertension following treatment, although not all will be azotemic.4 Feline target organs that can incur damage secondary to hypertension include the eye, kidney, central nervous system and cardiovascular system.4,6 The effects of hypertension on the eyes and heart have been described in cats.4,6,7 Hypertensive encephalopathy in the cat typically occurs following a precipitous and sustained rise in blood pressure that exceeds the limits of cerebral arterial autoregulation.1
Experimentally induced hypertensive encephalopathy in 2 cats was described.1 Macroscopic abnormalities included cerebellar herniation, external hemorrhages and widening and flattening of the cerebral gyri (edema). Histologic evaluation revealed severe pallor and rarefaction of the cerebral white matter with regional widening of periarteriolar spaces and accumulation of PAS positive protein droplets. Vascular lesions were described as arteriolar hyalinosis and hyperplastic arteriolosclerosis of pial arterioles. Rare ischemic changes and microhemorrhages were also found within the brain.1 The pathogenesis is thought to involve autoregulatory failure of cerebral arterial blood flow during elevations of blood pressure. Forced vessel overdistension leads to blood brain barrier breakdown, opening of endothelial tight junctions and leakage of plasma proteins into the extracellular space and formation of vasogenic edema. Vascular dilation often starts segmentally, but can become diffuse, leading to generalized, interstitial cerebral edema. Clinical signs of hypertensive encephalopathy in cats can include ataxia, lethargy, seizures, stupor, and blindness.1
In this case, hypertension was documented (200 mmHg), and the cat had a history of hyperthyroidism, mild azotemia, cardiomyopathy and moderate histologic lesions in the kidneys consistent with chronic renal disease. Based upon the ACVIM consensus guidelines for classification of blood pressure, this cat is in risk category IV, with severe risk of target organ damage.4 Thus, hypertension may have played a role in the formation of hyaline arteriopathy, however, the reason for the predilection in the CNS for the ventral spinal artery, basilar artery and branches is unknown. It is possible that thrombosis may have played a role in the formation of these changes, although similar vascular lesions were observed in the lungs and heart. The diameter of the ventral spinal artery in cats is the smallest at the level of C2, which is a potential predisposing factor for thrombosis.10 Evaluation of the brain did not reveal the histologic changes described in the experimentally induced hypertensive cats1 and histologic retinal arteriolar changes were not identified. Aneurysmal dilation of the ventral spinal artery has not been previously described as a sequel to hypertension in cats, however, ischemic lesions in the spinal cord of cats have been frequently reported.6,10,11 Predisposing medical conditions were reported to include chronic renal disease, hypertension, cardiomyopathy, and hyperthyroidism.10,11 In one case series of 19 cats with ischemic myelopathy, the most commonly affected regions of spinal cord included the C1-C5 (30%) and C6-T2 (30%) regions of the spinal cord, based upon MRI findings, with cervical spinal cord lesions in the region supplied by the ventral spinal artery.11
Although not present in this case, atherosclerosis is a lesion of arteriosclerosis in which fatty degenerative changes occur, and are typified by the presence of atheroma or fibrofatty plaque formation. In dogs, lipid is more frequently observed in the tunica media and adventitia, as opposed to the tunica intima in humans.7 Atherosclerotic susceptibility amongst animals is variable, with atherosensitive species including humans, rabbits, chickens and pigs, and atheroresistant species including dogs, cats, cattle, goats and rats. In dogs, atherosclerosis is almost always found in conjunction with endocrine diseases, most notably hypothyroidism and diabetes mellitus.7
Contributing Institution:
Animal Medical Center, 510 East 62nd St. New York, NY 10065
JPC Diagnosis: 1. Ventral spinal artery: Arteriosclerosis, diffuse, severe with aneurysmal dilatation, thrombosis, recanalization, and mural hyaline degeneration, and fibrosis.
2. Spinal cord, ventral funiculi: Axonal degeneration and loss, diffuse, mild to moderate.
JPC Comment: The contributor has provided an excellent review of hypertension and subsequent vascular change in cats. This particular case was subsequently published by Rylander et al.10 in 2014, as part of a case series involving 5 cats.
Vascular disease resulting from feline hypertension has been previously seen in the Wednesday Slide Conference in 2017 (hypertensive retinopathy), and 2010 (hypertensive encephalopathy). While a common thread of chronic renal disease is associated with most cases1 (and seen in this case), a number of other conditions may be responsible for hypertension in cats as well: hyperthyroidism (also noted in the clinical history in this case), diabetes mellitus, pheochromocytoma, hyperaldosteronism, and erythropoietin therapy.5
The pathogenesis of hypertensive encephalopathy is thought to
involve the development of vasogenic edema as a result of sudden increases in blood
pressure that exceed the autoregulatory capacity of the vasculature in the
brain, resulting in endothelial injury and breakdown of the blood-brain
barrier.5 A recent publication by the moderator2 has
detailed the systemic pathology and resultant vascular and parenchymal lesions
of feline hypertensive encephalopathy. In this case review of 12 cats, the
median age was 12 years, without apparent breed predilection. All 12 had
measured hypertension, from 16?300mm Hg. 11 of 12 animals had chronic tubulointerstitial
nephritis and 4/12 had concurrent hyperthyroidism (which is consistent with
previous publications). 6/12 had choroidal arteriopathy, and 5/12 had left ventricular
hypertrophy.2
Neurological signs were most often localized to the prosencephalon and/or
posterior fossa (brainstem and cerebellum), with 8/12 casts demonstrating
cranial neve deficits, 50% had altered mentation ranging from dull to comatose.
5/12 developed seizure activity.2
Gross lesions were only seen in 4/12 cases, to include cerebral edema with or
without displacement of the cerebellum. In this study, the primarily
histologic lesion was bilaterally symmetrical regional to diffuse cerebral
edema of the white matter, most severe at the dorsal aspect of white matter tracts.2
Areas of edema separated myelin sheaths, and were populated by subjectively
increased numbers of glial cells, including Alzheimer II astrocytes;
gemistocytic astrocytes were seen in 3/12 cases. Leptomeningeal
arteriosclerosis was identified in 9/12 cases, with 8 of 12 demonstrating
hyaline change. Lamellar fibrosis of the serosa (?onion-skinning?) was
relatively uncommon, being seen in 2 cases, one with the highest arterial
pressure, and one with the longest history of systemic hypertension.2
References:
1. Brown CA, Munday JS, Mathur S, Brown SA. Hypertensive encephalopathy in cats with reduced renal function. Vet Pathol. 2005; 42:642-649.
2. Church ME, Turek BJ, Durham AC. Neuropathology of spontaneous hypertensive encephalopathy in cats. Vet Pathol 2019; 56(5):778-782.
3. Fishbein GA, Fishbein MC. Arteriosclerosis, rethinking the current classification. Arch Pathol Lab Med. 2009; 133:1309-1316.
4. Jepson R. Feline systemic hypertension, classification and pathogenesis. J Fel Med Surg. 2011; 13:25-34.
5. Kent M. The cat with neurological manifestations of systemic disease; key conditions impacting on the CNS. Journal of Feline Med Surg. 2009;11:395-407.
6. Littman MP. Spontaneous systemic hypertension in 24 cats. J Vet Intern Med. 1994; 8:79-86.
7. Maggio F, DeFrancesco TC, Atkins CE, Pizzirani S, Gilger BC, Davidson MG. Ocular lesions associated with systemic hypertension in cats: 69 cases (1985-1998). J Am Vet Med Assoc. 2000; 217(5):695-702.
8. Maxie MG, Robinson WF. Cardiovascular system. In: Jubb, Kennedy, and Palmer?s Pathology of Domestic Animals, vol 3, 5th ed. New York, NY: Elsevier Limited; 2007:56-61.
9. Olsen JL. Hyaline arteriolosclerosis: new meaning for an old lesion. Editorial. Kidney Intl. 2003; 63:1162-1163.
10. Rylander H, Eminaga S, Palus V, Steinberg H, Caine A, Summers BA, Gehrke J, West C, Fox PR, Donovan TA, Cherubini GB. Feline ischemic myelopathy and encephalopathy secondary to hyaline arteriopathy in five cats. J Feline Med Surg 2014; 16(10):832-9.
11. Theobald A, Volk H, Dennis R, Berlato D, DeRisio L. Clinical outcome in 19 cats with clinical and magnetic resonance imaging diagnosis of ischaemic myelopathy (2000-2011). J Fel Med Surg. 2012; 15(2): 132-141.