CONFERENCE 25, CASE 3
Signalment:
8-year-old spayed female domestic shorthair cat (Felis catus).
History:
The patient presented for an approximately 72-hour progressive history of seizures manifesting as lip twitching, masticatory movements, excessive vocalization, blindness, and ptyalism. Interictal behavioral changes of restlessness, loss of environmental awareness and elevated pain as perceived by the owner were also reported. At first presentation (48 hours), a focal, full thickness ulcer was observed on the right cranial tongue. Physical examination was otherwise unremarkable. Complete blood cell count and blood chemistry were also unremarkable and the patient was sent home with robenacoxib and buprenorphine for pain control. At the final presentation (72 hours), the patient was reported to be hyperthermic and extremely agitated. While attempting to place an IV catheter for treatment, the patient underwent acute cardiac arrest.
Gross Pathology:
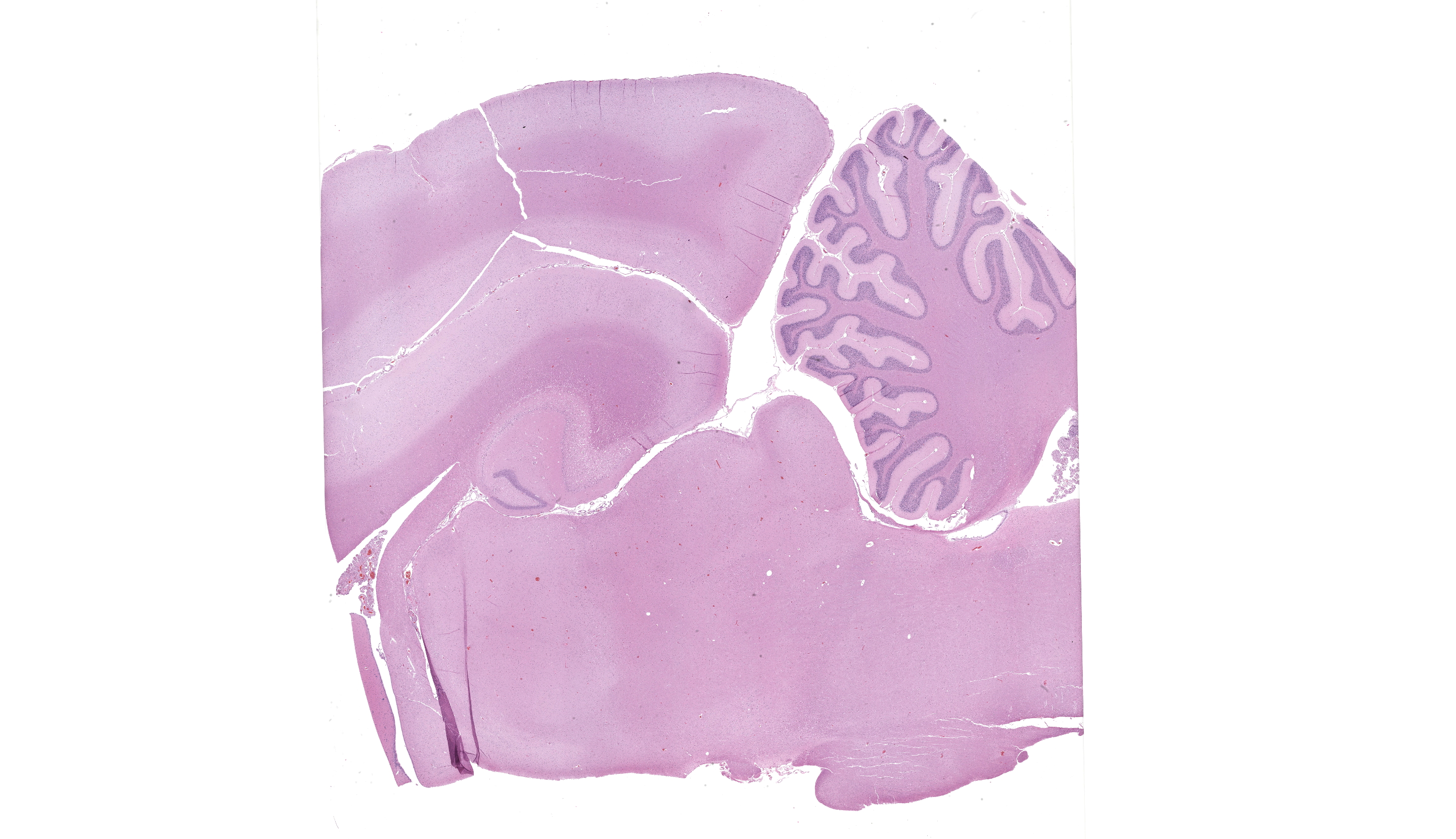
There were no remarkable gross findings within the formalin fixed brain. The right rostral tongue had a focal, linear, well-demarcated, purple to dark purple ulceration that measured 9 mm x 5 mm x 2 mm. The left lateral aspect of the tongue had a linear, red to dark red focus that measured 5 mm x 2 mm.
Laboratory Results:
Feline Herpes Virus - 1 PCR: Negative.
Snap FeLV/FIV Combo Test: Negative.
Microscopic Description:
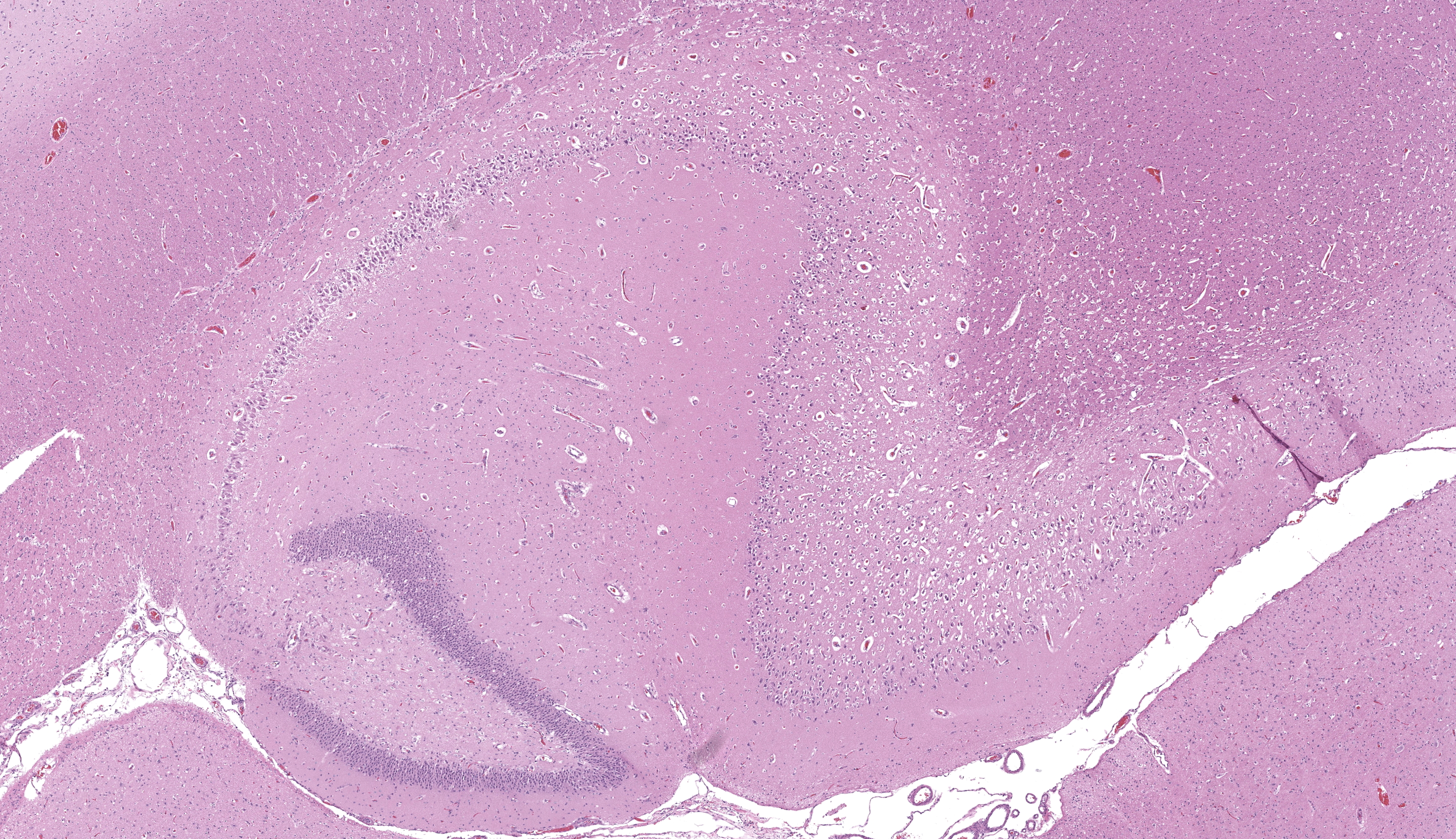
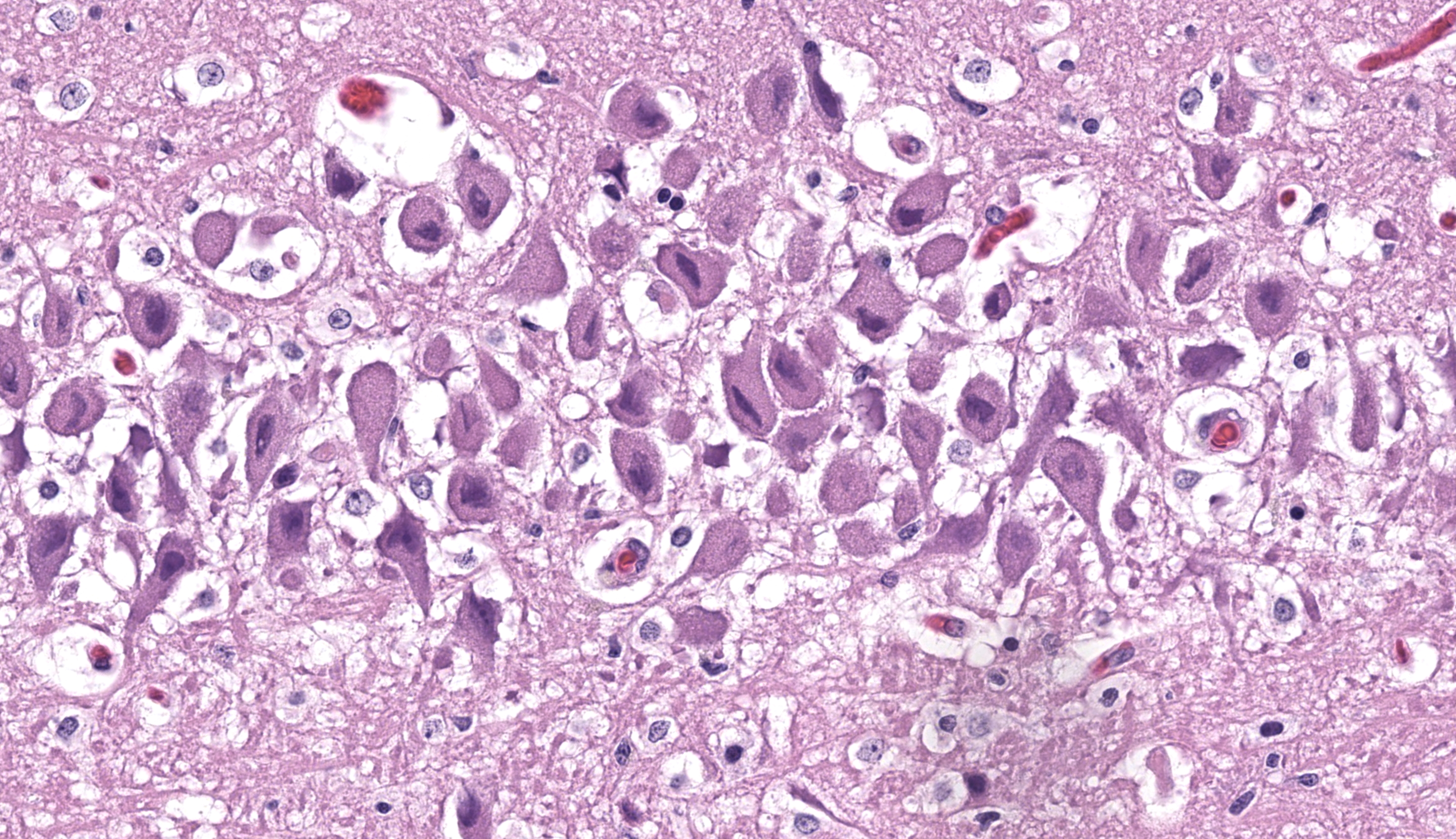
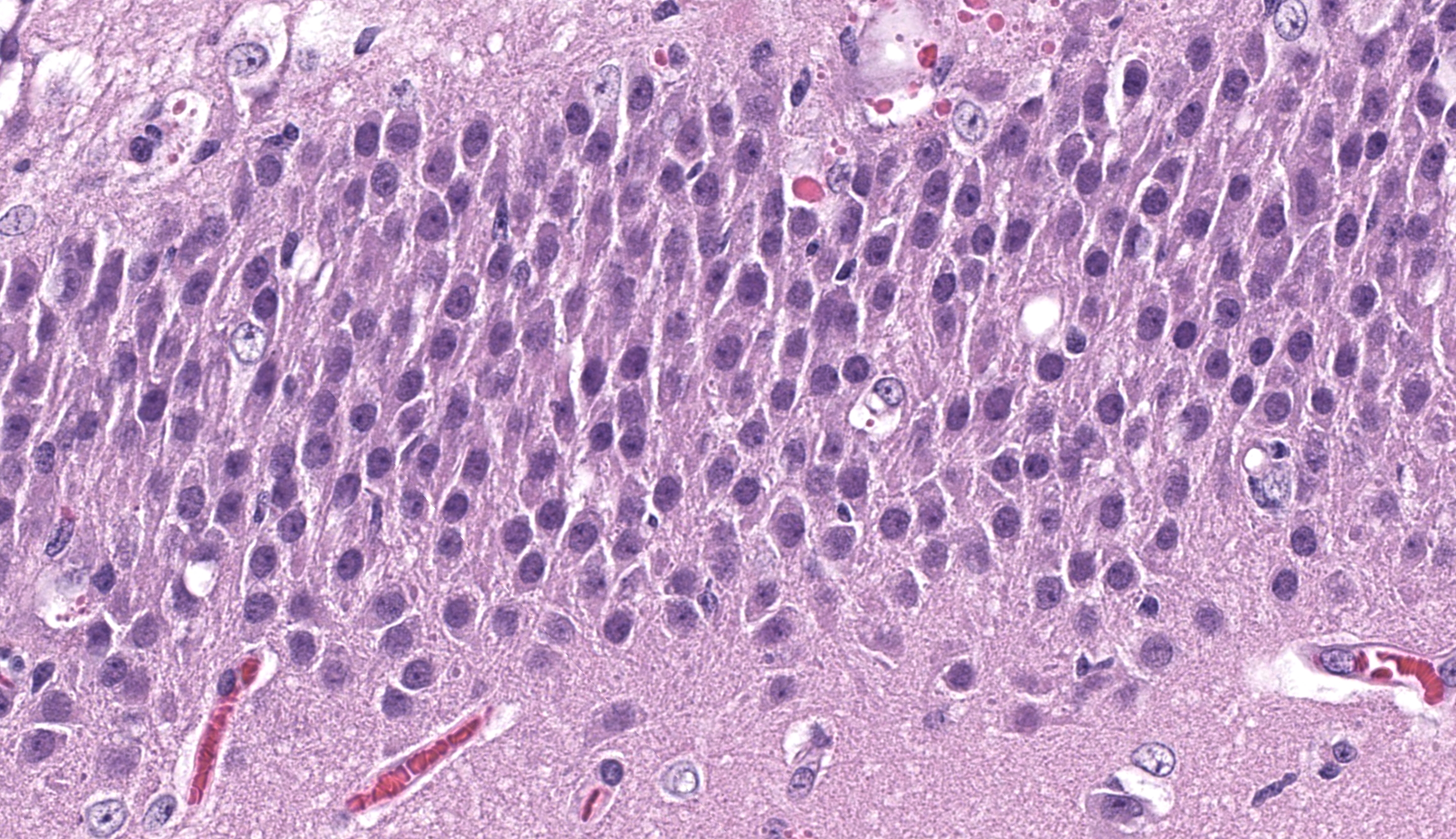
Cerebrum, hippocampus: Confined to the hippocampus and most prominent within CA1, scattered neurons are characterized by swelling, vacuolation, prominent nissl substance or are shrunken and angular with hypereosinophilic cytoplasm and fading or pyknotic nuclei. Glial cells are increased in number with frequent reactive astrocytes. Microglial cells frequently flank neuronal debris and are occasionally within degenerate and necrotic neurons (neuronophagia). There is proliferation of small caliber blood vessels lined by hypertrophic endothelium with rare perivascular aggregates of small numbers of mononuclear cells and edema. Neurons occasionally have basophilic glassy, nuclei.
Contributor’s Morphologic Diagnosis:
Cerebrum, hippocampus: Neuronal degeneration and necrosis, moderate to marked, regionally extensive and bilateral, acute with neuronophagia, astrocytosis, gliosis, microvascular proliferation, perivascular inflammation.
Other significant histological findings (slides not submitted):
Tongue: Full thickness ulceration, marked, multifocal, acute with necrosuppurative glossitis, muscular necrosis, neuritis and abundant intralesional bacterial colonies.
Condition: Feline hippocampal and piriform lobe necrosis (FHN)
Contributor’s Comment: A diagnosis of feline hippocampal and piriform necrosis (FHN) was given based on clinical presentation and histopathologic lesions consistent with those described in the literature and previous case reports. FHN is an acute onset neurologic disorder characterized clinically by rapidly progressive, often refractory focal, complex-partial and/or generalized tonic-clonic seizures. Interictal behavioral changes reflective of limbic dysfunction are common: loss of environmental awareness, agitation/aggression, hyperesthesia, ptyalism, pyrexia, urinary retention and hyperexcitability are described.5,7,8,15 FHN has been reported in several countries: Australia, Austria, England, Finland, Italy, Switzerland, and the United States.4,7,9,15,17,19,22 No sex, breed nor age disposition has been identified.
Neurologic examination and laboratory findings are non-specific. Therefore, clinical diagnosis is often by exclusion, coupling clinical presentation with Magnetic Resonance Imaging (MRI) findings (the hippocampus and piriform lobes are T2 and FLAIR hyperintense, T1 hypointense, +/- contrast enhancement).4,7,9,15,17,19,22 Gross examination of the brain is regularly unremarkable, however, accentuated vascular structures or mild malacic changes in the piriform lobe and hippocampus have been described.1,7 Definitive diagnosis of FHN is made via histopathology. Microscopically, FHN is characterized by varying degrees of acidophilic neuronal necrosis confined to the hippocampus and piriform lobe structures. The distribution of lesions is bilateral and symmetric, and tend to be most prominent within CA1. Microvascular proliferation, microgliosis/neuronophagia and astrocytosis are also common features. Lymphohistiocytic perivascular inflammation, and central chromatolysis may also be present, though less frequently.5,7
Aforementioned, the exact pathogenesis of FHN is yet to be elucidated. Epileptogenic, immune-mediated, infectious, toxic, vascular, structural, degenerative and paraneoplastic processes have all been proposed.3,4,7,9,10,15,19,20,22 Given the shared clinical presentation and histologic findings of FHN across a potpourri of suggested etiologies, some authors hypothesize that FHN is more suggestive of a pathogenic neurolocalization as opposed to a specific entity.19 Hippocampal pyramidal neurons are particularly susceptible to endogenous excitotoxicity, so it seems conceivable that alterations in metabolism, function and/or architecture of this neuroanatomical region could uncouple the regulatory mechanisms of neurotransmitter metabolism (hypothesized to be glutamate and/or aspartate),2,5 leading to regionally extensive edema, ischemia and necrosis.
The scope of comparative pathology of the FHN phenotype is relatively narrow. Bilateral ischemic changes have been reported in dogs with refractory epilepsy and/or prolonged seizures.11,12 However, the topic represents an exciting area of immunology and neuropathology research. The clinical presentation and histologic findings described in FHN are similar to those described in human Autoimmune Limbic Encephalitis (ALE).3,10,13,20 Many cases of ALE are defined by aberrant production of antibodies against the neuronal cell membrane antigens.3,10,13,16,20,21 Antibodies against voltage-gated potassium channel complex (VGKC) extracellular domains of leucine-rich glioma-inactivated 1 (LGI1) and contactin-associated protein-like 2 (CASPR2) are some of the most prevalently isolated, which together have been defined as LG1 encephalitis. Voltage-gated potassium channels (VGKCs) represent a group of signaling proteins capable of modulating a wide variety of synaptic functions, most prominently neuronal excitability and neurotransmitter release. Dysfunction or damage to the VGKC complex carries the potential of hyperexcitability, seizure and resulting excitotoxicity. In veterinary literature, recent studies have demonstrated the presence of anti-LGI1 antibodies in cats with clinical presentations, MRI findings and histologic hippocampal necrosis/sclerosis that parallel human LG1 encephalitis. Clinically, this constellation of findings has been termed feline complex partial seizures with orofacial involvement (FEPSO). FEPSO may represent an immune-mediated etiology of FHN, and carries the potential to serve as a spontaneously occurring animal model of human LG1 encephalitis.3,10,19
Unfortunately, feline epilepsy is often enigmatic as many epileptic cats have atypical seizures, genetic markers for feline epilepsy are poorly described, and a complete neurologic work up is often not performed.17 Therefore, these challenges hinder a more thorough depiction of the processes underlying the FHN phenotype, resulting in ambiguous diagnoses which muddle antemortem characterization and histopathologic correlation (when available). Fortunately, growing awareness of FHN as a component in feline epilepsy will hopefully chaperone further exploration and clarification on the likely heterogeneous FHN phenotype.
In the presented case, several neurons had smudgy to glassy, basophilic appearance prompting PCR for feline herpesvirus-1, which was negative. Thus, the histogenesis remains unclear. FeLV/FIV status for this animal was negative and the cat was up to date on vaccinations and was housed solely indoors, decreasing suspicions for rabies and/or Toxoplasma spp. The ulceration observed on the tongue was attributed as a sequelae to an unobserved seizure.
Contributing Institution:
Colorado State University Veterinary Diagnostic Laboratory
Fort Collins, CO
https://vetmedbiosci.colostate.edu/vdl/
JPC Morphologic Diagnosis: Hippocampus: Neuronal degeneration and necrosis, segmental, with reactive astrocytosis and spongiosis.
JPC Comment: Although the lesion in this case is somewhat understated relative to the other slides in this conference, the contributor’s summary is anything but, and wonderfully summarizes the current literature on this entity (and we appreciate the callback to previous WSC!). The sagittal sectioning of this brain provides another opportunity to check for autolytic change, using the granular and molecular layer interface as a good anchoring point – these layers should be in apposition with little vacuolation and condition of cerebellar neurons should also be evaluated.23 The slide is well-preserved with little artifact present.
The hippocampal changes in this case are better appreciated with a matched animal control, or at least, review of pertinent veterinary literature24 to brush up on the various subfields of the cornu ammonis (CA). Neuronal necrosis within CA1 was appreciable as shrunken, eosinophilic cells with pyknotic nuclei, though Dr. Koehler reminded conference participants that the ventral hippocampus can also have darker neurons as an artifact secondary to tissue manipulation and excessive pressure, and that these should be carefully distinguished. Other corroborating evidence such as reactive astrocytes (with increased cytoplasm, peripheralized chromatin, and occasional doublets representing division) also confirmed that changes observed were real. We captured this distinction of ‘reactive’ in our morphologic diagnosis as we feel that it emphasizes not only the cellular changes of astrocytes, but the increased cell number elicited by this process.
This case also had good examples of simultaneous cytotoxic and vasogenic edema. This is possibly attributed to seizure episodes, though glutamate effects are more localized to CA3 and changes in this cat were attenuated. Likewise, seizure episodes might also generate lesions in other portions of the brain (e.g. head trauma and hemorrhage) which are absent in this section, though the effects of global hypoxia and cytokine generation might explain vascular-centered edema in this brain. We also briefly discussed the smudgy inclusions within neurons noted by the contributor – these likely reflect clumped protein aggregates and cellular dysfunction (unfolded protein response and integrated stress response)) and should not be confused with viral inclusion bodies, to include Negri bodies from rabies virus.18
References:
- The Joint Pathology Center. Feline hippocampal necrosis. JPC Wednesday Slide Conference; 2013-2014, Conference 22, Case 2. https://www.askjpc.org/wsco/wsc_showcase2.php?id=MjRJVGMxMm9vVFR1clh1Zm4yeTRWZz09.
- Barker-Haliski M, White HS. Glutamatergic Mechanisms Associated with Seizures and Epilepsy. Cold Spring Harb Perspect Med. 2015 Jun 22;5(8):a022863.
- Binks S, Lamquet S, Crawford AH, Meurs A, Irani SR, Pakozdy A. Parallel roles of neuroinflammation in feline and human epilepsies. Vet J. 2022 Dec;290:105912.
- Brini E, Gandini G, Crescio I, Fatzer R, Casalone C. Necrosis of hippocampus and piriform lobe: clinical and neuropathological findings in two Italian cats. J Feline Med Surg. 2004 Dec;6(6):377-81.
- Cantile C., Youssef S. Nervous System. (2016). In: Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, ed. Maxie M.G. 6th edition. Philadelphia: Elsevier, vol 1, p 398-400.
- Dow, S. W., Poss, M. L. and Hoover, E. A. (1990): Feline immunodeficiency virus: a neurotropic lentivirus. J. Acquir. Immune Defic. Syndr. 3, 658–668.
- Fatzer R, Gandini G, Jaggy A, Doherr M, Vandevelde M. Necrosis of hippocampus and piriform lobe in 38 domestic cats with seizures: a retrospective study on clinical and pathologic findings. J Vet Intern Med. 2000 Jan-Feb;14(1):100-4.
- Fletcher NF, Meeker RB, Hudson LC, Callanan JJ. The neuropathogenesis of feline immunodeficiency virus infection: barriers to overcome. Vet J. 2011 Jun;188(3):260-9.
- Fors S, Van Meervenne S, Jeserevics J, Rakauskas M, Cizinauskas S. Feline hippocampal and piriform lobe necrosis as a consequence of severe cluster seizures in two cats in Finland. Acta Vet Scand. 2015 Jul 28;57(1):41.
- Glantschnigg-Eisl U, Klang A, Kneissl S, Lang B, Waters P, Irani SR, Binks SNM, Pakozdy A. A feline model of spontaneously occurring autoimmune limbic encephalitis. Vet J. 2023 Jun-Jul;296-297:105974.
Hasegawa D, Fujita M, Nakamura S, Takahashi K, Orima H (2002) Electrocorticographic and histological findings in a shetland sheepdog with intractable epilepsy. J Vet Med Sci 64:277–279. - Hasegawa D, Nakamura S, Fujita M, Takahashi K, Orima H (2005) A dog showing Klüver–Bucy syndome-like behavior and bilateral limbic necrosis after status epilepticus. Vet Neurol Neurosurg J 7:1–14.
13. Hasegawa D, Ohnishi Y, Koyama E, Matsunaga S, Ohtani S, Nakanishi A, Shiga T, Chambers JK, Uchida K, Yokoi N, Fukata Y, Fukata M. Deleted in colorectal cancer (netrin-1 receptor) antibodies and limbic encephalitis in a cat with hippocampal necrosis. J Vet Intern Med. 2019 May;33(3):1440-1445. - Hora AS, Tonietti PO, Guerra JM, Leme MC, Pena HF, Maiorka PC, Brandão PE. Felid herpesvirus 1 as a causative agent of severe nonsuppurative meningoencephalitis in a domestic cat. J Clin Microbiol. 2013 Feb;51(2):676-9.
- Klang A, Högler S, Nedorost N, Weissenbacher-Lang C, Pákozdy Á, Lang B, Weissenböck H. Hippocampal necrosis and sclerosis in cats: A retrospective study of 35 cases. Acta Vet Hung. 2018 Jun;66(2):269-280.
- Montojo MT, Petit-Pedrol M, Graus F, Dalmau J. Clinical spectrum and diagnostic value of antibodies against the potassium channel related protein complex. Neurologia. 2015 Jun;30(5):295-301.
- Moore SA. Seizures and epilepsy in cats. Vet Med (Auckl). 2014 Jul 30;5:41-47. doi: 10.2147/VMRR.S62077. PMID: 32670845; PMCID: PMC7337200.
- Nietfeld JC, Rakich PM, Tyler DE, Bauer RW. Rabies-like inclusions in dogs.J Vet Diagn Invest.1989;1(4):333-338.
- Pakozdy A, Gruber A, Kneissl S, Leschnik M, Halasz P, Thalhammer JG. Complex partial cluster seizures in cats with orofacial involvement. J Feline Med Surg (2011) 13:687–93. 10.1016/j.jfms.2011.05.014
- Pakozdy A, Halasz P, Klang A, Bauer J, Leschnik M, Tichy A, Thalhammer JG, Lang B, Vincent A. Suspected limbic encephalitis and seizure in cats associated with voltage-gated potassium channel (VGKC) complex antibody. J Vet Intern Med. 2013 Jan-Feb;27(1):212-4. doi: 10.1111/jvim.12026. Epub 2012 Dec 28. PMID: 23278981.
- Plantone D, Renna R, Koudriavtseva T. Neurological diseases associated with autoantibodies targeting the voltage-gated potassium channel complex: immunobiology and clinical characteristics. Neuroimmunology and Neuroinflammation. 2016; 3: 69-78.
- Scalia B, Caine A, Pittaway R, Cherubini GB. Feline temporal lobe epilepsy: seven cases of hippocampal and piriform lobe necrosis in England and literature review. J Feline Med Surg. 2022 Jun;24(6):596-608.
- Wohlsein P, Deschl U, Baumgärtner W. Nonlesions, unusual cell types, and postmortem artifacts in the central nervous system of domestic animals. Vet Pathol. 2013 Jan;50(1):122-43.
- Zilli J, Schänzer A, Büttner K, Kressin M, Schmidt MJ (2022) Quantitative and qualitative evaluation of the hippocampal cytoarchitecture in adult cats with regard to the pathological diagnosis of hippocampal sclerosis. PLOS ONE 17(5): e0268010.