CONFERENCE 23, CASE 1
Signalment:
2-year-old male white-faced saki monkey (Pithecia pithecia)
History:
A privately owned 2-year-old male white-faced saki monkey (Pithecia pithecia) was brought to the University of Tennessee College of Veterinary Medicine’s zoo medicine service for hindlimb lameness. Radiographs identified generalized osteopenia and a folding fracture of the right tibia. The monkey had been housed exclusively indoors since birth.
Gross Pathology:
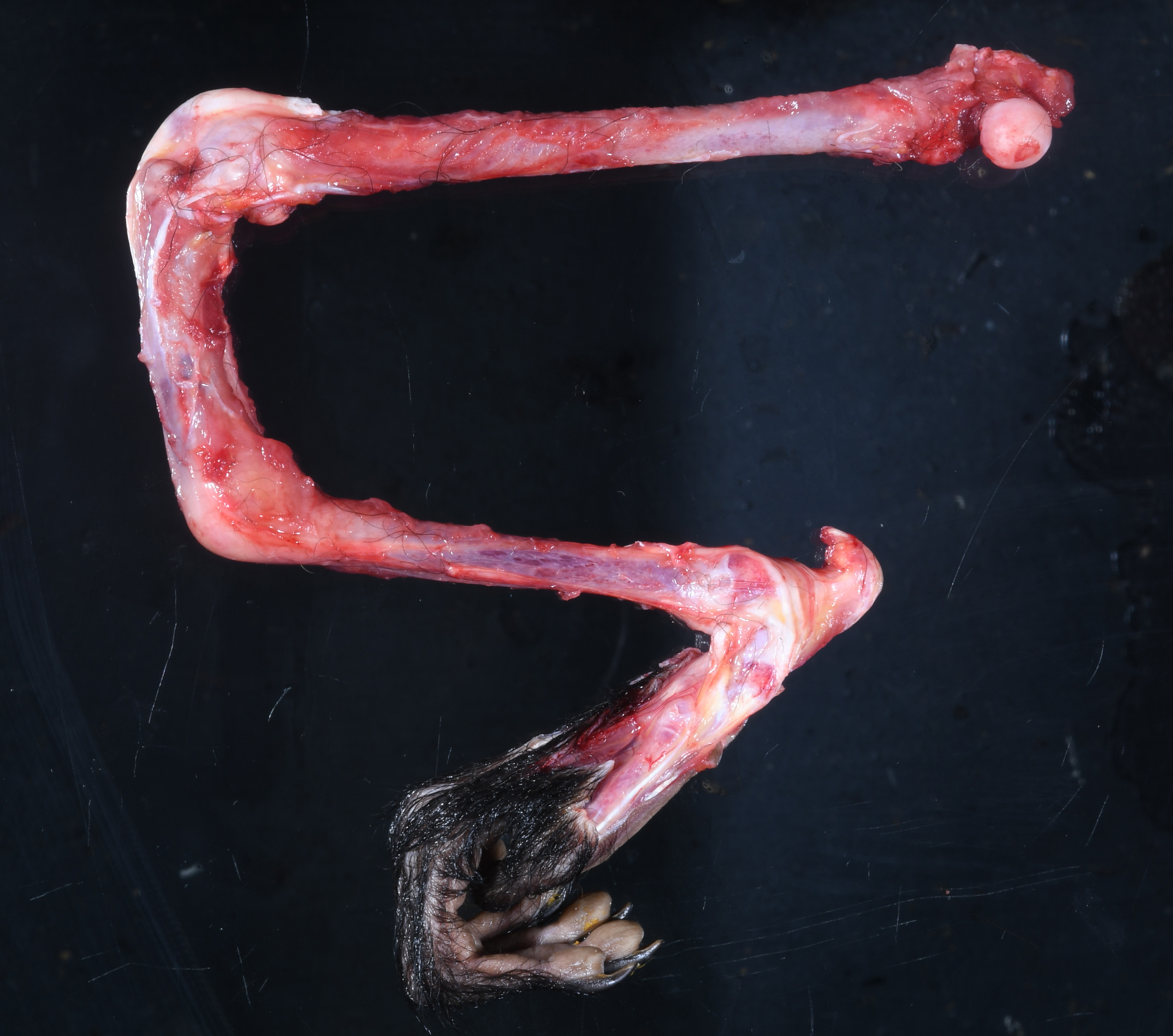
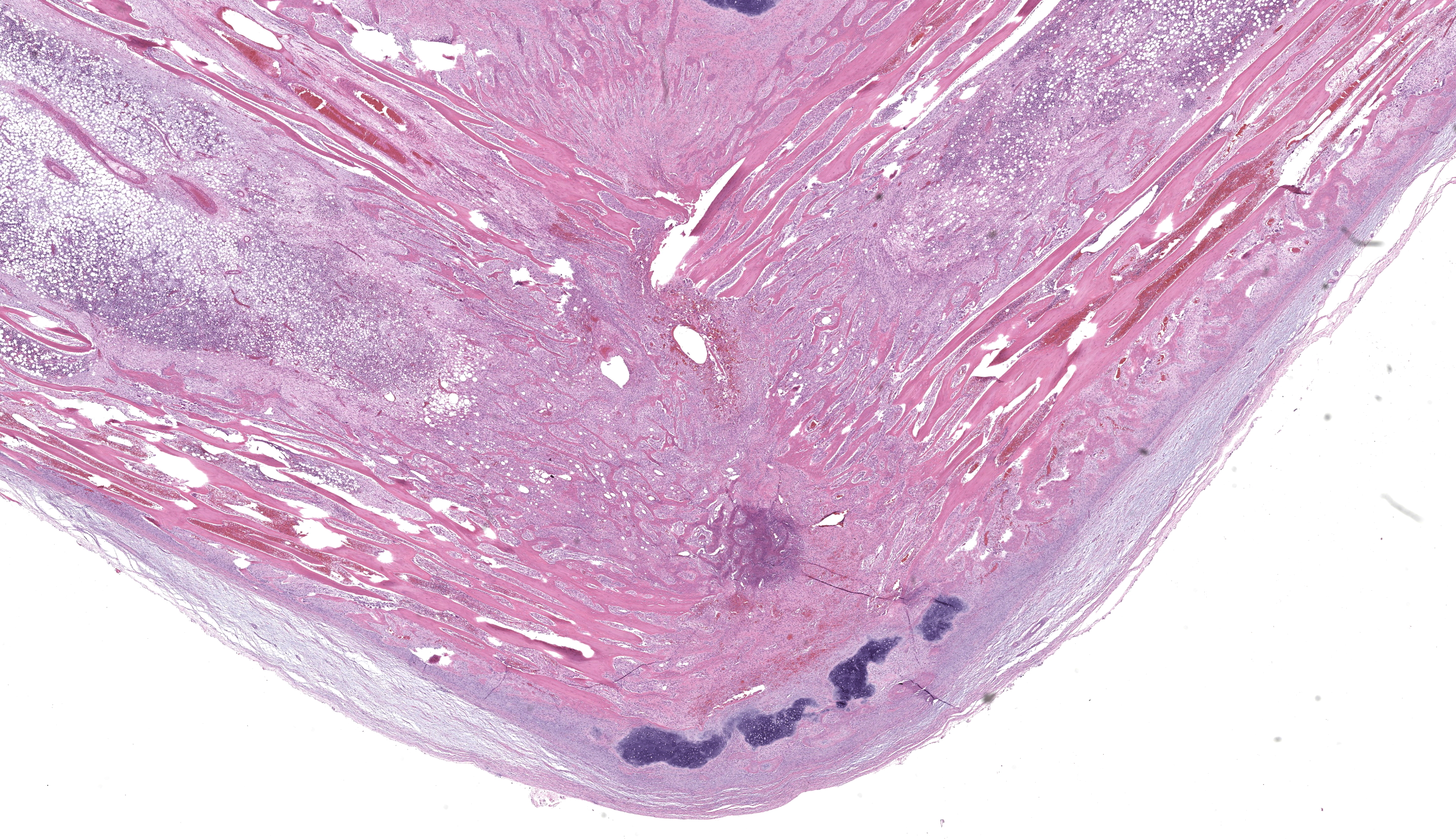
Midway along the tibial diaphysis of the right hindlimb, approximately 5.5 cm distal to the stifle, was a 3 cm in diameter, circumferential, white, firm, boney proliferation (callus). The tibia and fibula immediately distal to the callus were bent and directed approximately 70 degrees caudally.
On cut section, there was a 2 cm diameter rim of periosteum, skeletal muscle, and subcutis immediately surrounding the callus which was dark red to black (hemorrhage) and had a translucent yellow wet gelatinous appearance (edema). Bone marrow was dark red, soft, and floated in 10% neutral buffered formalin.
Laboratory Results:
A serum chemistry panel identified severely elevated alkaline phosphatase (ALP) at 4167 u/L and mild hypocalcemia with 0.9 mmol/L ionized calcium.
Microscopic Description:
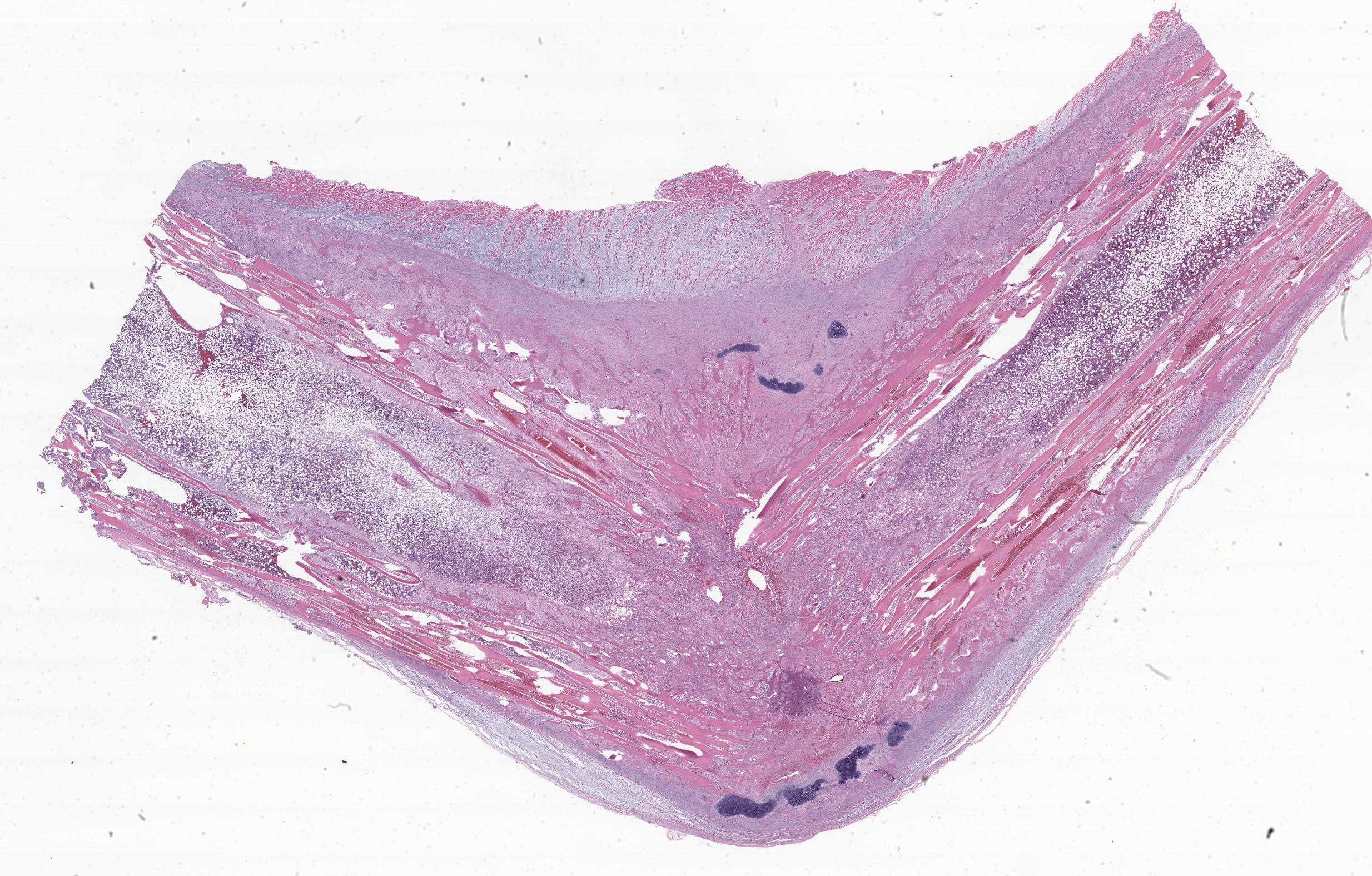
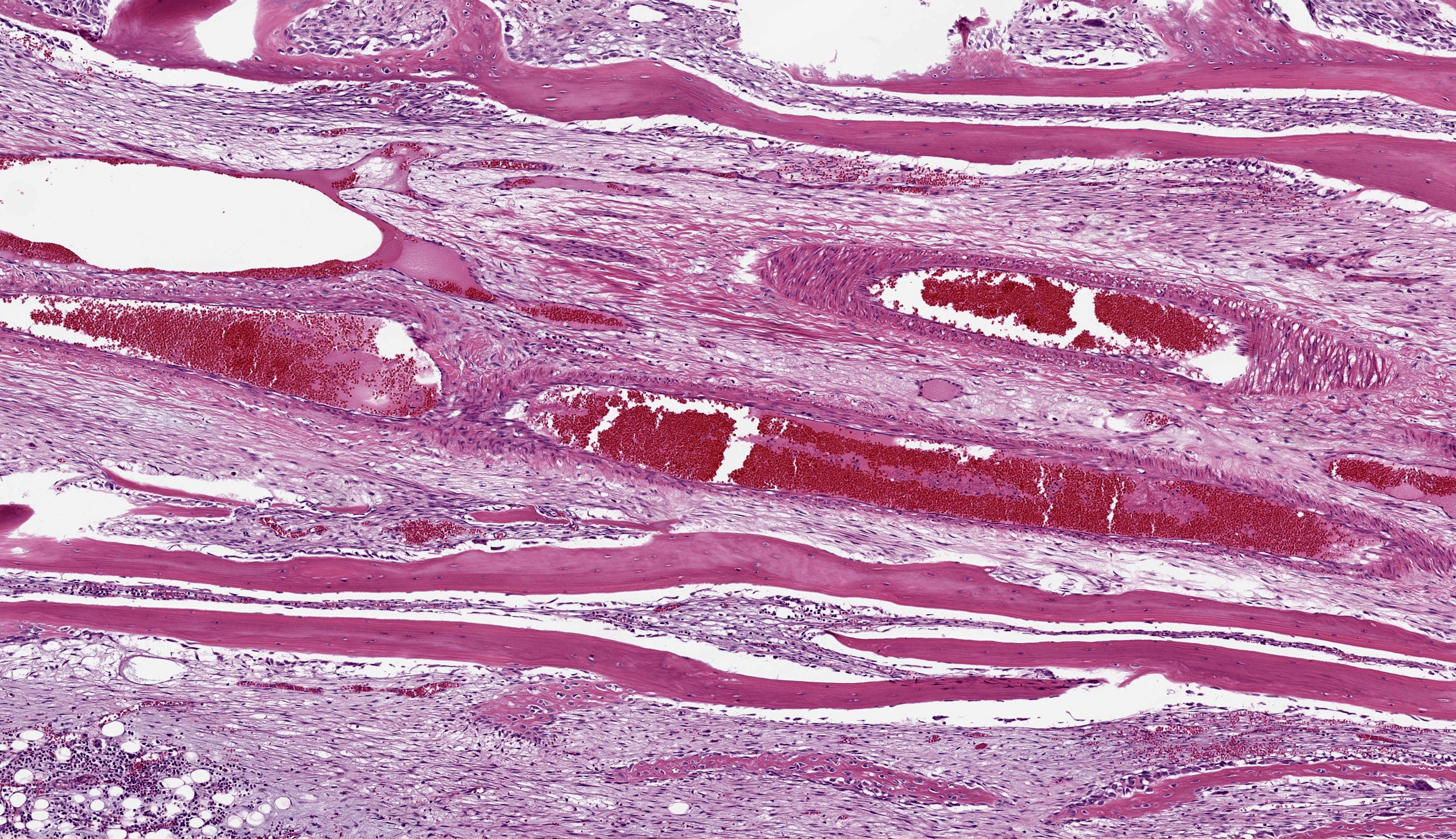
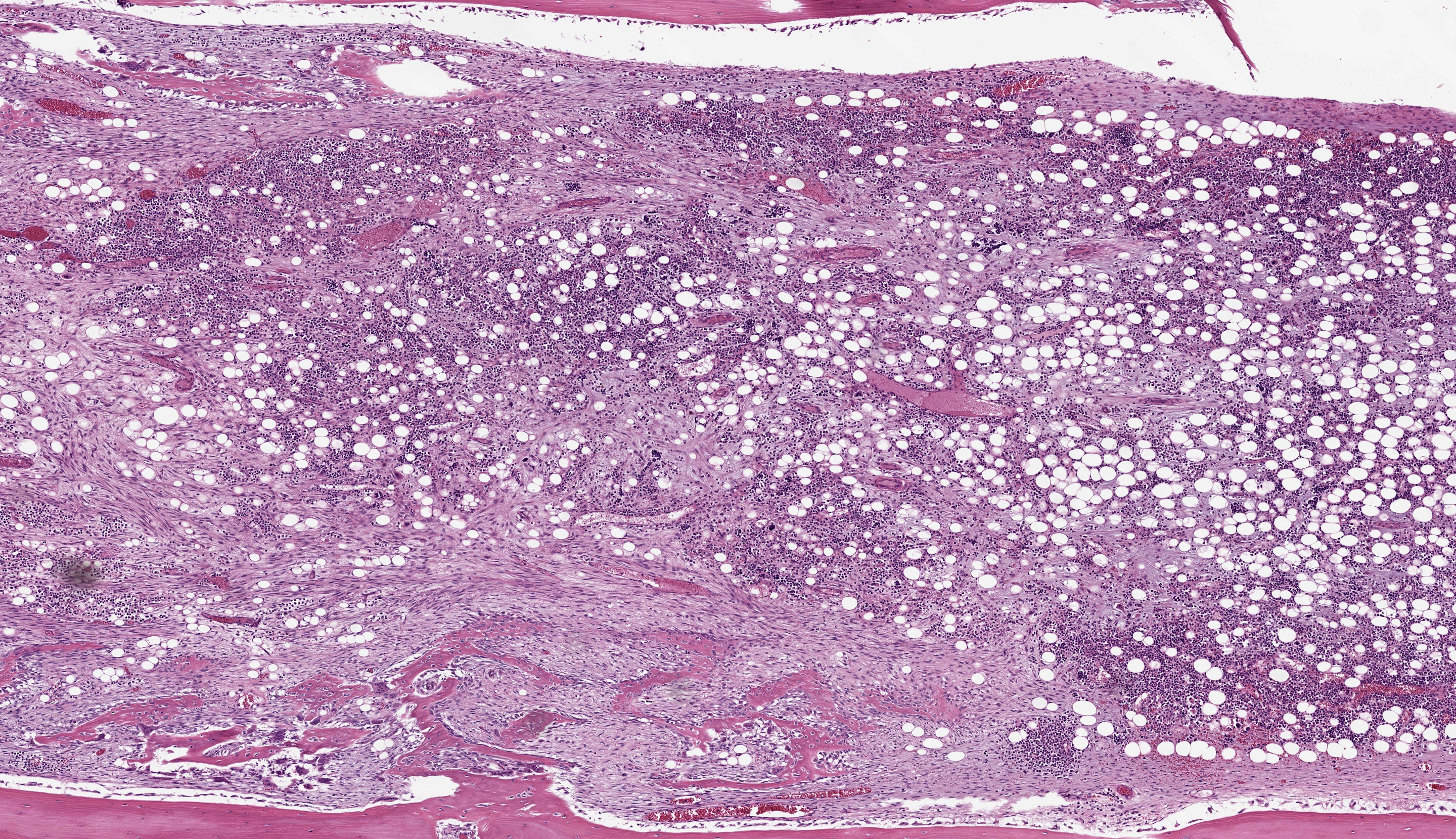
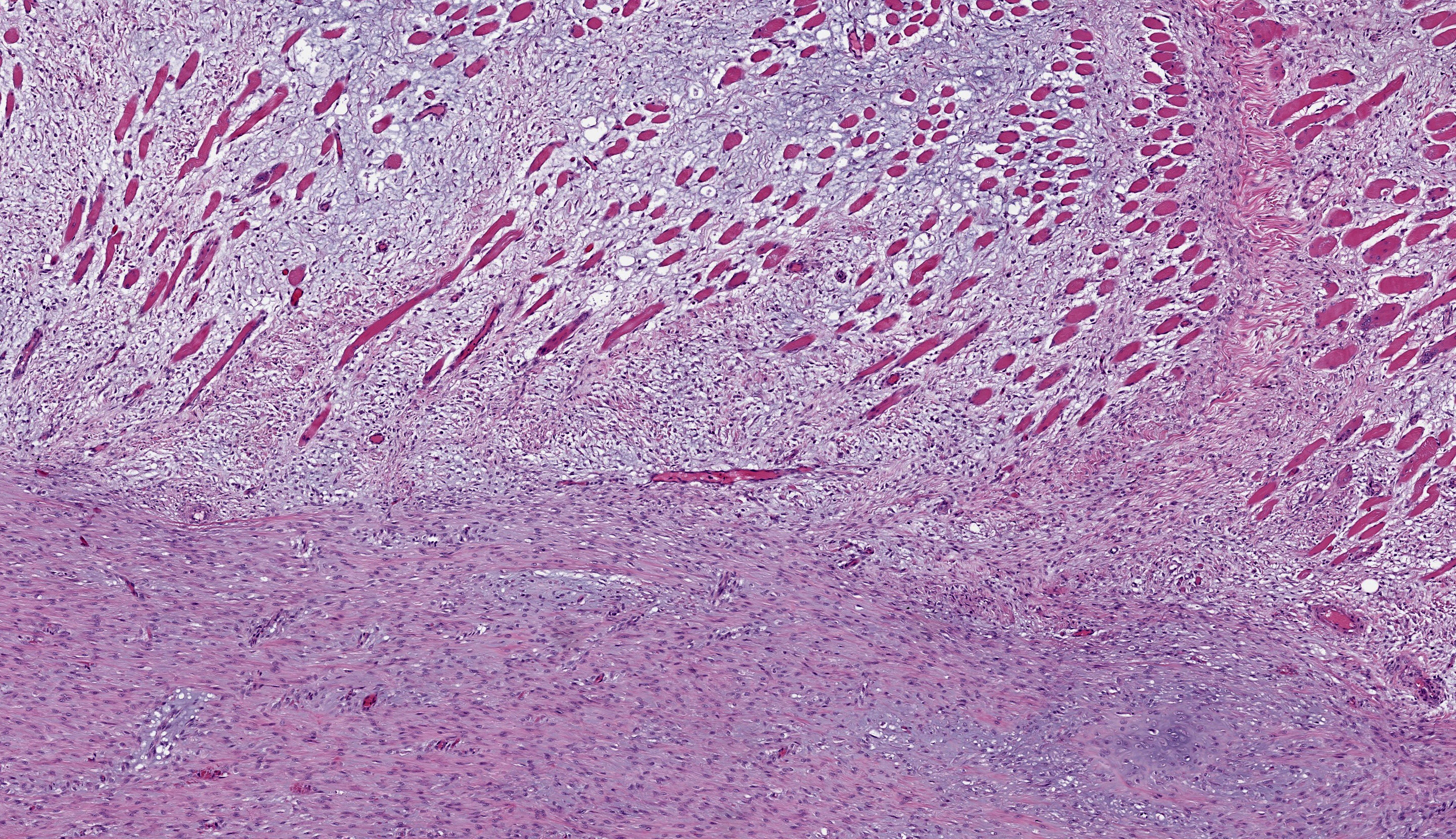
Right tibia: The cortical bone is thin, discontinuous, and replaced by loose fibrovascular tissue (fibroplasia). Fragments of cortical bone are surrounded by fibrovascular tissue and segmentally lined by a single layer of osteoblasts or increased numbers of osteoclasts within Howship’s lacunae (resorption). Thereare bands of thick fibrovascular tissue expanding the periosteal and endosteal margins with frequent islands and irregular trabeculae of immature woven bone lined by osteoblasts. There is a focal complete cortical defect and the long axes of bone are positioned at approximately 90 degrees (fracture). At the fracture site, cortical bone is surrounded and separated by the previously described fibrovascular connective tissue mixed with erythrocytes transitioning to basophilic cartilaginous matrix with chondrocytes and irregular trabeculae of woven bone. The marrow cavity has approximately 50% cellularity with megakaryocytes, and erythroid and myeloid precursors of various developmental stages. A lightly basophilic myxomatous matrix extends into and surrounds adjacent skeletal muscle bundles which are frequently small and rounded (atrophy).
Contributor’s Morphologic Diagnosis:
Right tibia: Severe chronic diffuse fibrous osteodystrophy with a diaphyseal fracture and callus
Contributor’s Comment:
This white-faced saki monkey (Pithecia pithecia) had nutritional secondary hyperparathyroidism with fibrous osteodystrophy and a pathologic tibial fracture. In this case, radiographs also showed a generalized diffuse decrease in radiodensity of all bones, indicating osteopenia.
Metabolic bone disease includes multiple morphologic entities which can occur in combination in the same individual. These include rickets, osteomalacia, osteoporosis, and fibrous osteodystrophy, all caused by deficiencies or imbalances in vitamin D, calcium, and/or phosphorous.1
Fibrous osteodystrophy is characterized by extensive bone resorption, proliferation of fibrous tissue, and the formation of poorly mineralized immature bone.1 The pathogenesis includes an elevation of parathyroid hormone (PTH) which may be caused by primary hyperparathyroidism, secondary hyperparathyroidism, or hypercalcemia of malignancy. Primary hyperparathyroidism may be caused by a functional parathyroid neoplasm (adenoma or adenocarcinoma) and secondary hyperparathyroidism may be caused by either renal disease or nutritional imbalances of calcium, phosphorous, and/or vitamin D.
Vitamin D may be obtained from the diet and radiation of the skin with ultraviolet light. Metabolic bone disease is not uncommon in privately owned non-human primates, as their diets may be inadequately balanced, they often receive insufficient exposure to ultraviolet light, and lack supplementation of their diet with vitamin D3 (cholecalciferol).3 New World monkeys have specific vitamin D requirements to maintain serum calcium levels and are thus exquisitely sensitive to calcium and vitamin D deficiencies.5 A lack of exposure to ultraviolet light impedes the conversion of 7-dehydrocholesterol in the skin to pre-vitamin D3.4 Pre-vitamin D3 is then isomerized to vitamin D3 (cholecalciferol). Forms of vitamin D from the diet and skin undergo hydroxylation in the liver, forming 25-hydroxycholecalciferol or 25-hydroxyvitamin D [25(OH)D], which is the form of vitamin D in the circulation. This is then hydroxylated in the kidney by 1α-hydroxylase to form calcitriol (1, 25-dihydroxyvitamin D), the metabolically active form of vitamin D.2
Although the diet is unknown, the history of being housed exclusively indoors likely led to vitamin D deficiency and nutritional hyperparathyroidism in this monkey. The parathyroids had diffuse chief cell hyperplasia consistent with secondary hyperparathyroidism. There was no clinical, gross, or histologic evidence of renal disease.
Contributing Institution:
University of Tennessee College of Veterinary Medicine
2407 River Drive
Knoxville, TN 37996
https://vetmed.tennessee.edu/
JPC Morphologic Diagnosis:
1. Long bone: Osteoclast-mediated bone resorption, marked, with cortical fibrosis, pathologic fracture, and callus.
Skeletal muscle: Atrophy, multifocal, marked.
JPC Comment: This week’s moderator was Dr. Brian Murphy from the University of California at Davis, who selected four bone and oral pathology-centered cases to dazzle conference participants. This first case is beautifully sectioned with a characteristic gross photo that drew an audible reaction from the group. Participants discussed metabolic bone disease at length with Dr. Murphy and used slide features to draw inferences which we faithfully attempt to capture below.
Foremost, evaluation of bone should start first from subgross magnification. While we approach all of our descriptions in this manner, Dr. Murphy emphasized the importance of a careful survey of the cortex, periosteum, endosteum, marrow elements, and surrounding tissue before jumping to higher magnifications. Likewise, evaluation of bone ALWAYS requires radiographs and a detailed clinical history to support interpretation. In this case, the presence of a large, well-developed callus is helpfully centered by the contributor and provides a clue for the apparent right-angle turn in the direction of the bone. That said, the callus is the effect and not the cause of the lesion in this saki monkey (although it is impressive). Evaluation of adjacent cortical bone reveals the answer, in that it is both wide and lytic with the presence of numerous osteoclasts and fibrous tissue – these features together yield the diagnosis of fibrous osteodystrophy. Dr. Murphy noted that in chronic cases, the presence of osteoclasts may be minimal or absent, though the diagnosis can still be made. The atrophy of skeletal muscle in this case is also distinctive and is itself another effect – we considered the possibility of both disuse and abnormal weight-bearing as contributory factors.
In addition, evaluation of metabolic bone disease can reveal multiple concurrent processes with overlapping histologic features which the pathologist should expect. In young animals, fibrous osteodystrophy can co-exist with rickets, which warrants consideration of the physis for disorganization and expansion of the zone of hypertrophy as well as evaluation of the metaphysis for unmineralized osteoid.1 The cortex is generally thickened with unmineralized osteoid along the periosteum. In adult animals, osteomalacia also reflects inability to mineralize osteoid, but there is no open physis to consider. There may be decreased numbers of thin bony trabeculae and/or cortical thinning.1 Osteoporosis (a clinical syndrome reflecting pain, lameness, and/or bony deformation) may also be superimposed on cases of metabolic bone disease due to microfracture and infraction (fracture without cortical displacement) of bone. Before rendering a final diagnosis, it is helpful to summarize all associated slide features and weigh whether one or more processes is occurring that could explain the constellation of histologic signs.
References:
- Craig LE, Dittmer KE, Thompson KG. Bones and joints. In: Maxie, MG ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals.6th 1. St. Louis, MO: Elsevier; 2016:60-80.
- Dittmer KE, Thompson KG. Vitamin D Metabolism and Rickets in Domestic Animals: A Review. Veterinary Pathol. 2011;48(2):389-407.
- Hatt JM, Sainsbury AW. Unusual case of metabolic bone disease in a common marmoset (Callithrix jacchus). Veterinary Rec. 1998;143(3):78-80.
- Minich DJ, Henry BA, Levens GP. Metabolic bone disease in a white-faced saki (Pithecia pithecia). Vet Rec Case Rep. 2022;10:e393
- Olson EJ, Shaw GC, Hutchinson EK, et al. Bone Disease in the Common Marmoset: Radiographic and Histological Findings. Veterinary Pathol. 2015;52(5):883-893.