Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 12
December 13th, 2017
CASE IV: O291/16 (JPC 4101762).
Signalment: 8 month-old, female, intact, Pug, Canis familiaris, canine.
History: The dog had two generalized seizures within a week. On clinical examination, the dog presented with abnormal posture and right-sided circling. The dog also had generalized muscle twitching, most notably in the face. Menace response was absent. The dog was subsequently admitted to the animal hospital for intensive care treatment with phenobarbital, glucocorticosteroids and clindamycin, after which it became semi-comatose. As symptoms did not subside, the dog was humanely euthanized.
Gross Pathology: The dog was brought directly to necropsy. No gross findings were evident.
Laboratory results:
Hematology, biochemistry, electrolytes and C-reactive protein (CRP) were within normal reference ranges. Toxoplasma serology was negative.
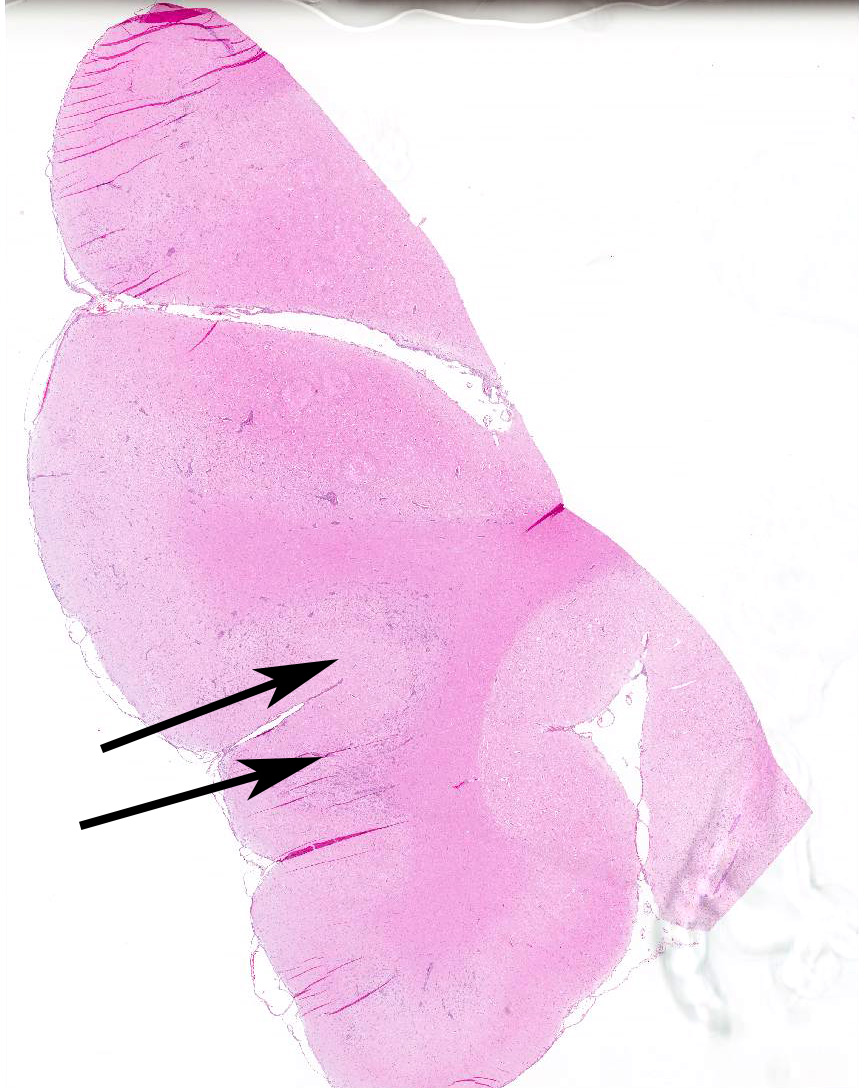
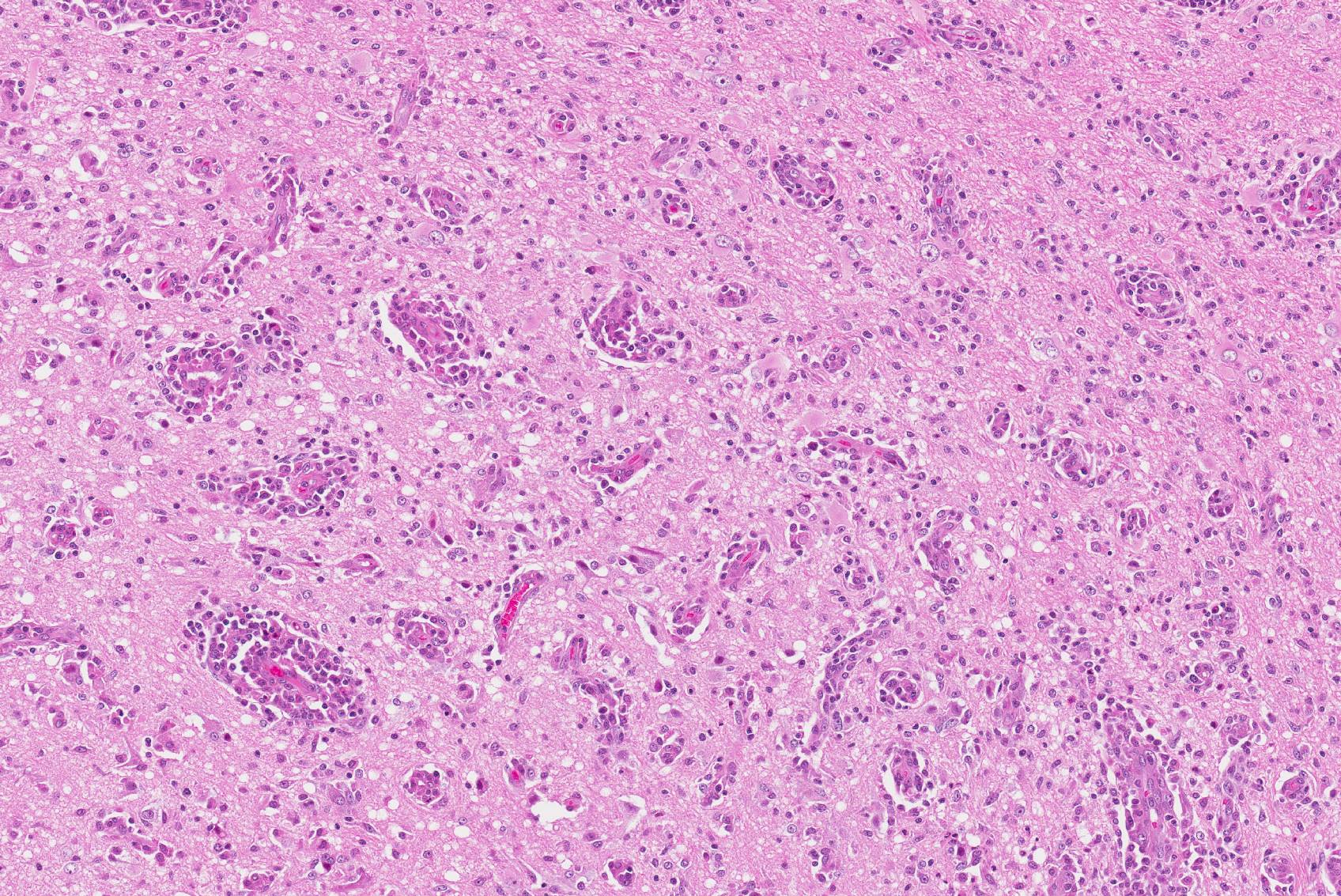
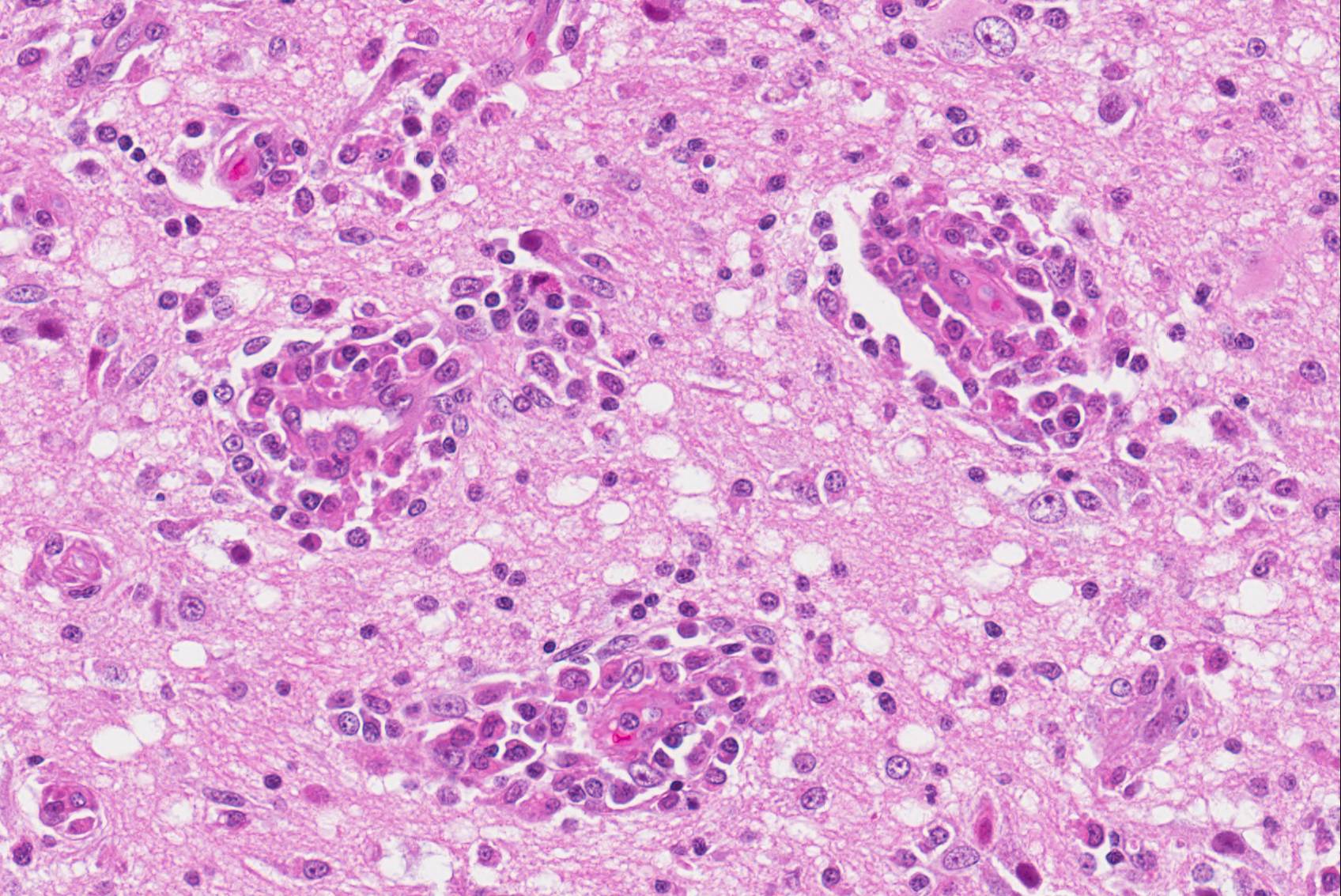
Microscopic Description: Brain, parietal cortex: In the grey-white matter interface, with predominance in the cortical grey matter and sparing of deep cortical white matter, there are multifocal to diffuse inflammatory lesions, distributed within the neuropil and centered around perivascular spaces (perivascular cuffs). The neuronal parenchyma exhibits moderate to severe vacuolation (rarefaction) with scattered glial cells and vessels outlined by hypertrophic endothelium. Perivascular inflammatory infiltrates are composed primarily of lymphocytes, plasma cells, macrophages and occasional binucleated cells. These cells can also be seen in the leptomeninges and around meningeal vessels.
Multifocally, phagocytic cells engulfing neuronal debris (neuronophagia) are evident (not in all slides), as well as neurons exhibiting pyknotic nuclei, with shrunken cellular outline and hypereosinophilic cytoplasm (neuronal necrosis). Glial nodules and diffuse gliosis can be seen throughout the aforementioned areas, as well as neuronal loss and satellitosis. Clefting of perivascular spaces from surrounding parenchyma is also observed (perivascular edema).
Contributor’s Morphologic Diagnosis:
Brain: Polioencephalitis and meningitis, non-purulent and necrotizing, multifocal to coalescing, severe.
Name the Condition: Necrotizing meningoencephalitis
Contributor’s Comment: Necrotizing meningoencephalitis (NME), formerly known as “pug dog encephalitis”, is an idiopathic disorder primarily affecting small breed dogs such as Pugs, and less commonly Pekingese,3 Chihuahua,8 Maltese,3 Shih Tzu and other small breed species4. Dogs present with neurological signs at ages ranging between 6 months to 7 years, with a mean age of onset at 29 months.18 Affected Pug dogs often present at a median age of 18 months, and the disease is most often seen in young females.9 Occasional reports of NME in large breed dogs exist, but is a rare occurrence.5 Affected dogs usually present with sudden onset of prosencephalic clinical signs including seizures and depression, often with fatal outcome.18
A commonly discussed differential diagnosis of NME is granulomatous meningoencephalomyelitis (GME). GME also affects small breed dogs, such as terriers and toy breeds, but may occur in larger breeds as well, with an age range of 6 months to 12 years.3
The anatomical distribution of lesions in NME and GME varies. NME usually affects the cortical grey matter, with relative sparing of deeper periventricular tissues. The lesions are often confluent over large areas, and may be evident grossly (but not always). Multifocal swelling and yellow foci of malacia can be observed bilaterally, but asymmetrically, in the cerebral hemispheres. GME usually produces milder gross changes, but granulomatous foci are occasionally seen.17 Another variant of idiopathic necrotizing meningoencephalitis is necrotizing leukoencephalitis (NLE). NLE is a condition primarily affecting Yorkshire terriers, Boston terriers and Chihuahuas.3 As for NLE, malacic foci are observed but, in contrast to NME, are centered in the white matter of the cerebral hemispheres.
Histologically, NME primarily affects the cortical grey matter. Areas of rarefaction, vacuolation and neuronal necrosis are significant features of the disease, accompanied by non-purulent inflammatory infiltrates of lymphocytes, plasma cells and macrophages, arranged diffusely and as perivascular cuffs.3 In contrast, histologic changes in GME are multifocal and patchy, with lesions centered in the basal parts of the brain, particularly the white matter of the brain stem and spinal cord.18 GME often produces a more cellular infiltrate consisting of numerous macrophages and giant cells, with or without mitotic activity.3 These infiltrates may be arranged in a granulomatous fashion with formation of epithelioid cells.17 However, large malacic foci are rarely seen in GME, and for this reason, GME may be hard to distinguish from brain malignant histiocytosis.3
Histopathological evaluation is required to distinguish between all subsets of idiopathic meningoencephalitis.18
A definitive etiology has not been identified for either NME or GME. For NME and GME, immune-mediated reactions against brain tissue have been suggested, including anti-astrocyte antibodies in NME and GME,10 and anti-GFAP antibodies specifically in NME patients.11 GFAP has been found to be one of the more commonly targeted auto-antigens in Pug dogs.16 According to immunohistochemical studies, a predominance of CD3- positive T-cells has been detected in GME.12 CD163-positive cells (macrophages) can be detected in both NME and GME,12 but lysozyme immunoreactive cells are more abundant in NME than GME.17 However, the distribution of the macrophage cell population varies from diffuse in NME to granulomatous and perivascular in GME.12 On protein level, a marked increase of IFN-γ and IL-17 has been observed in NME and GME, respectively.13
The inheritance pattern of NME has been investigated. Two loci have been identified in NME-affected dogs, one of these being associated with dog leukocyte antigen class II.2, 7 Common genetic backgrounds have been proposed in dogs with NME regardless of breed. However, the implication of these traits may differ between dog breeds.15 In Pug dogs specifically, a strong familial inheritance pattern has been demonstrated.6
Infectious etiologies for these encephalitides have also been discussed. According to one study, Mycoplasma canis has been identified in some cases of NME and GME (4/25 and 1/25 respectively).1 However, the role of Mycoplasma in the pathogenesis of these diseases remains uncertain. Regarding viral causes, alpha-herpesviruses are proposed as possible agents.3 However, no association between NME and viral pathogens such as herpes-, adeno- or parvoviruses has been found in many studies, including one pertaining to approximately 5000 studied individuals.1,6,14
JPC Diagnosis: Cerebrum: Meningoencephalitis, lymphohistiocytic, multifocal to coalescing, moderate with edema, neuronal loss, and astrocyte hypertrophy, Pug (Canis familiaris), canine.
Conference Comment: Necrotizing meningoencephalitis (NME, fully described above) results in malacia of the cerebral cortex and mononuclear cell infiltration in the meninges and perivascular spaces that is bilateral but asymmetrical and mostly affecting the gray matter. The main differential, granulomatous meningoencephalitis (GME), is characterized by histiocytic inflammation predominately within the cerebral white matter that can form granulomas with chronicity.3
Both NME and GME have currently unidentified causes and are active areas of research. Many recent works are detailed above, and a recent study in pug dogs reviewed individuals that had undergone extrahepatic portosystemic shunt attenuation surgery to identify if there were increased postoperative neurologic complications, including NME. Only four dogs were necropsied, none of which had evidence of NME.20
In this case, attendees were not certain that this represented a case of NME as no definitive areas of necrosis were present in the submitted slide. Given the lack of demonstrable parenchymal necrosis and the extent of concurrent white matter lesions, conference participants discussed the possibility that this may actually case represent a case of GME. Another topic of discussion not mentioned by the contributor is the presence of numerous clustered hypertrophic astrocytes clustered which demonstrated large vesicular nuclei and abundant pink cytoplasm. Participants discussed whether the term “Alzheimer type 2 astrocytes” was appropriate in this case, a term has been traditionally reserved for reactive astrocytes in hepatic encephalopathies which are induced by accumulation of ammonia and other endogenous toxins, or cases involving cerebral amyloid.3 Preferring to be conservative in such matters which are known to enflame true neuropathologists, the “hypertrophic astrocytes” was settled upon in this case.
Contributing Institution:
Swedish University of Agricultural Sciences
Department of Biomedical Sciences and Veterinary Public Health, Section of Pathology
BOX 7028
SE 750 07, Uppsala, Sweden
https://www.slu.se/en/departments/biomedical-sciences-veterinary-public-health/
References:
- Barber RM, Porter BF, Li Q, et al. Broadly reactive polymerase chain reaction for pathogen detection in canine granulomatous meningoencephalomyelitis and necrotizing meningoencephalitis. J Vet Intern Med. 2012;26(4):962-968.
- Barber RM, Schatzberg SJ, Corneveaux JJ, et al. Identification of risk loci for necrotizing meningoencephalitis in Pug dogs. J Hered. 2011;102, Suppl 1:S40-46.
- Cantile C, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol. 1. 6th Philadelphia, PA:Saunders Elsevier; 2016:262, 344, 392-394.
- Cooper JJ, Schatzberg SJ, Vernau KM, et al. Necrotizing meningoencephalitis in atypical dog breeds: a case series and literature review. J Vet Intern Med. 2014;28(1):198-203.
- Estey CM, Scott SJ, Cerda-Gonzalez S. Necrotizing meningoencephalitis in a large mixed-breed dog. J Am Vet Med Assoc. 2014;245(11):1274-1278.
- Greer KA, Schatzberg SJ, Porter BF, et al. Heritability and transmission analysis of necrotizing meningoencephalitis in the Pug. Res Vet Sci. 2009;86(3):438-442.
- Greer KA, Wong AK, Liu H, et al. Necrotizing meningoencephalitis of Pug dogs associates with dog leukocyte antigen class II and resembles acute variant forms of multiple sclerosis. Tissue Antigens. 2010;76(2):110-118.
- Higgins RJ, Dickinson PJ, Kube SA, et al. Necrotizing meningoencephalitis in five Chihuahua dogs. Vet Pathol. 2008;45(3):336-346.
- Levine JM, Fosgate GT, Porter B, et al. Epidemiology of necrotizing meningoencephalitis in Pug dogs. J Vet Intern Med. 2008;22(4):961-968.
- Matsuki N, Fujiwara K, Tamahara S, et al. Prevalence of autoantibody in cerebrospinal fluids from dogs with various CNS diseases. J Vet Med Sci. 2004;66(3):295-297.
- Matsuki N, Takahashi M, Yaegashi M, et al. Serial examinations of anti-GFAP autoantibodies in cerebrospinal fluids in canine necrotizing meningoencephalitis. J Vet Med Sci. 2009;71(1):99-100.
- Park ES, Uchida K, Nakayama H, . Comprehensive immunohistochemical studies on canine necrotizing meningoencephalitis (NME), necrotizing leukoencephalitis (NLE), and granulomatous meningoencephalomyelitis (GME). Vet Pathol. 2012;49(4):682-692.
- Park ES, Uchida K, Nakayama H, .Th1-, Th2-, and Th17-related cytokine and chemokine receptor mRNA and protein expression in the brain tissues, T cells, and macrophages of dogs with necrotizing and granulomatous meningoencephalitis. Vet Pathol. 2013;50(6):1127-1134.
- Schatzberg SJ, Haley NJ, Barr SC, et al. Polymerase chain reaction screening for DNA viruses in paraffin-embedded brains from dogs with necrotizing meningoencephalitis, necrotizing leukoencephalitis, and granulomatous meningoencephalitis. J Vet Intern Med. 2005;19(4):553-559.
- Schrauwen I, Barber RM, Schatzberg SJ, et al. Identification of novel genetic risk loci in Maltese dogs with necrotizing meningoencephalitis and evidence of a shared genetic risk across toy dog breeds. PLoS One. 2014;9(11):e112755.
- Shibuya M, Matsuki N, Fujiwara K, et al. Autoantibodies against glial fibrillary acidic protein (GFAP) in cerebrospinal fluids from Pug dogs with necrotizing meningoencephalitis. J Vet Med Sci. 2007;69(3):241-245.
- Suzuki M, Uchida K, Morozumi M, et al. A comparative pathological study on canine necrotizing meningoencephalitis and granulomatous meningoencephalomyelitis. J Vet Med Sci. 2003;65(11):1233-1239.
- Talarico LR, Schatzberg SJ. Idiopathic granulomatous and necrotizing inflammatory disorders of the canine central nervous system: a review and future perspectives. J Small Anim Pract. 2010;51(3):138-49.
- Uchida K, Park E, Tsuboi M, Chambers JK, Nakayama H. Pathological and immunological features of canine necrotizing meningoencephalitis and granulomatous meningoencephalitis. Vet J. 2016;213:72-77.
- Wallace ML, MacPhail CM, Monnet E. Incidence of postoperative neurologic complications in pugs following portosystemic shunt attenuation surgery. J Am Anim Hosp Assoc. 2017 Nov 13:Epub ahead of print. doi: 10.5326/JAAHA-MS-6534.