CASE 4: 19A087 (JPC 4135350-00)
Signalment:
2.8 year-old, male, Indian-origin rhesus macaque (Macaca mulatta)
History:
This animal was part of an SIV vaccine trial and had received multiple immunizations prior to being challenged with pathogenic SIV. Physical exam findings revealed mild dehydration, facial swelling, left-sided facial paralysis and diarrhea. After 3 weeks with no response to treatment humane euthanasia was elected.
Gross Pathology:
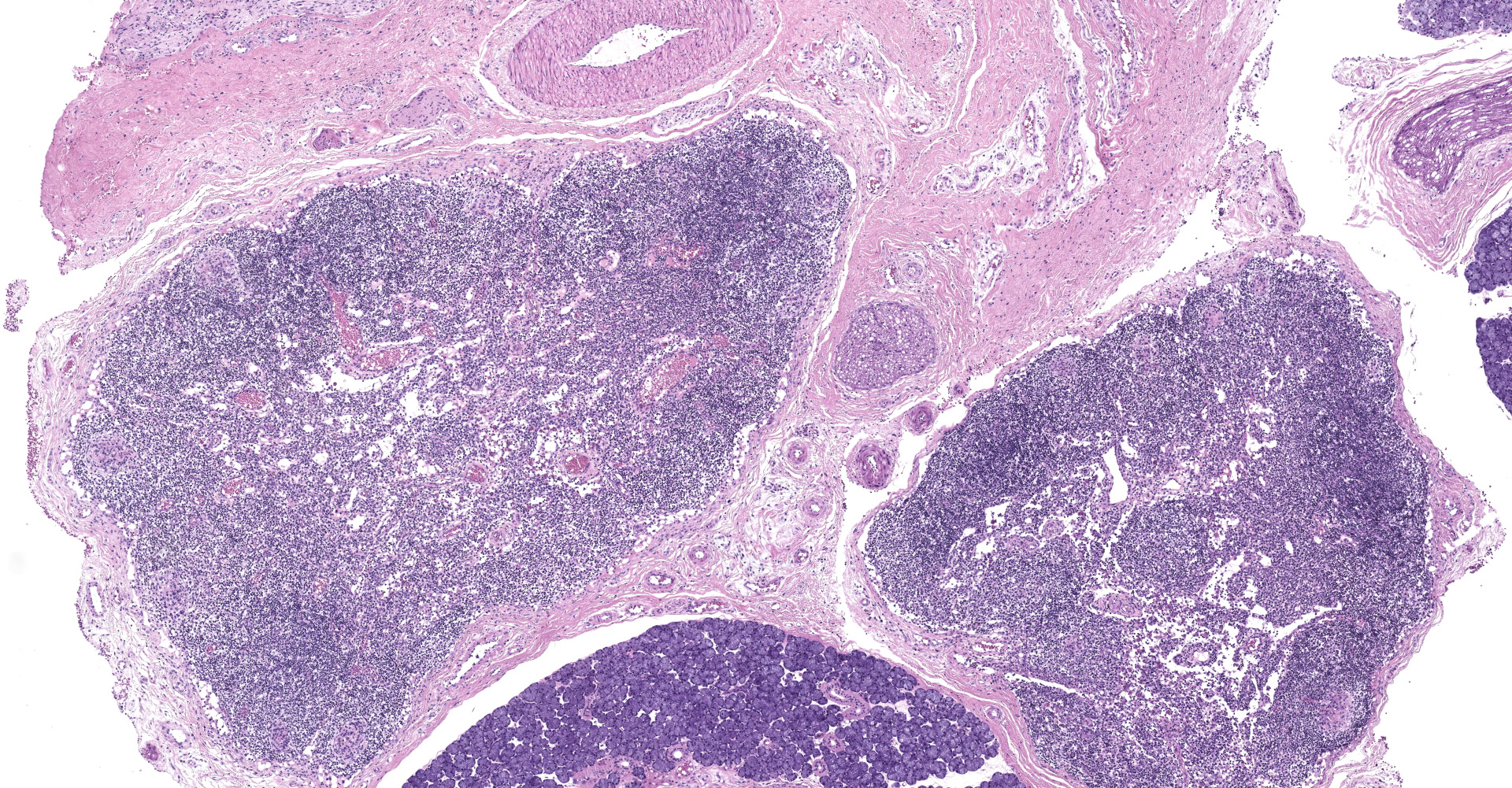
Presented for postmortem examination is a 2.8 year-old, male, Indian-origin rhesus macaque. There is moderate, left-sided fascial swelling. On cut surface the subcutis is expanded by moderate edema. The left trigeminal ganglia are enlarged and reddened compared to the right. The mucosa of the gastric antrum is diffusely reddened. The duodenum, jejunum, and ileocecal junction are similarly reddened. The colon contains copious, liquid, fetid feces.
Laboratory results:
|
Complete Blood Count |
||
|
|
Value |
Reference |
|
Wbc |
18.3 |
6.6-15.5 |
|
Rbc |
5.9 |
4.1-7.8 |
|
Hgb |
12.8 |
10.1-15.9 |
|
Hct |
40.9 |
34.8-55.2 |
|
Mcv |
69.9 |
63.7-86.9 |
|
Mch |
21.9 |
19.1-27.7 |
|
Mchc |
31.3 |
28.9-35.4 |
|
Rdw |
13.8 |
10.9-15.3 |
|
Plt |
476000 |
193-676 |
|
Mpv |
10.7 |
|
|
%Neu |
89.9 |
20.6-46.9 |
|
%Lym |
5.2 |
35.2-84.1 |
|
%Mon |
4.4 |
1.8-9.9 |
|
%Eos |
0.2 |
|
|
%Bas |
0 |
|
|
#Neu |
16.5 |
|
|
#Lym |
1 |
|
|
#Mon |
0.8 |
|
|
#Eos |
0.2 |
|
|
#Bas |
0 |
|
|
Chemistry Panel |
||
|
|
Value |
Reference |
|
Na |
145 |
136-145 |
|
K |
4.1 |
3.5-5.1 |
|
Cl |
3.6 |
98-107 |
|
Pro |
5.2 |
6.4-8.9 |
|
Alb |
3.1 |
3.5-5.7 |
|
Glob |
2.1 |
1.9-3.9 |
|
A/G |
1.5 |
0.5-3.5 |
|
BUN |
33 |
25-Jul |
|
Glu |
5 |
70-105 |
|
Crt |
0.2 |
0.6-1.3 |
|
Ast |
116 |
13-39 |
|
Alt |
76 |
|
|
Bn/Cr |
220 |
|
|
|
|
|
|
Csf pro |
132 |
|
Microscopic description:
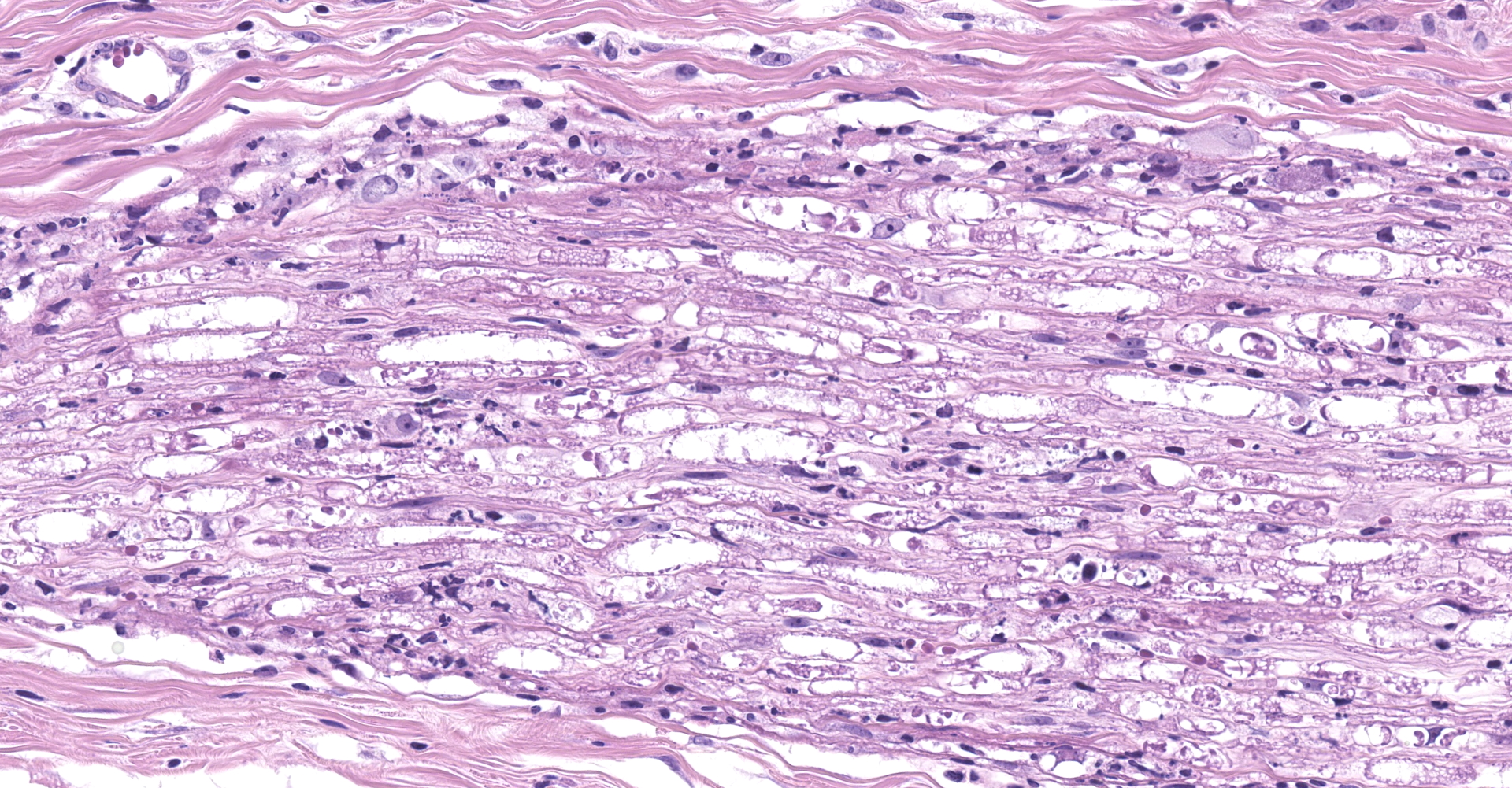
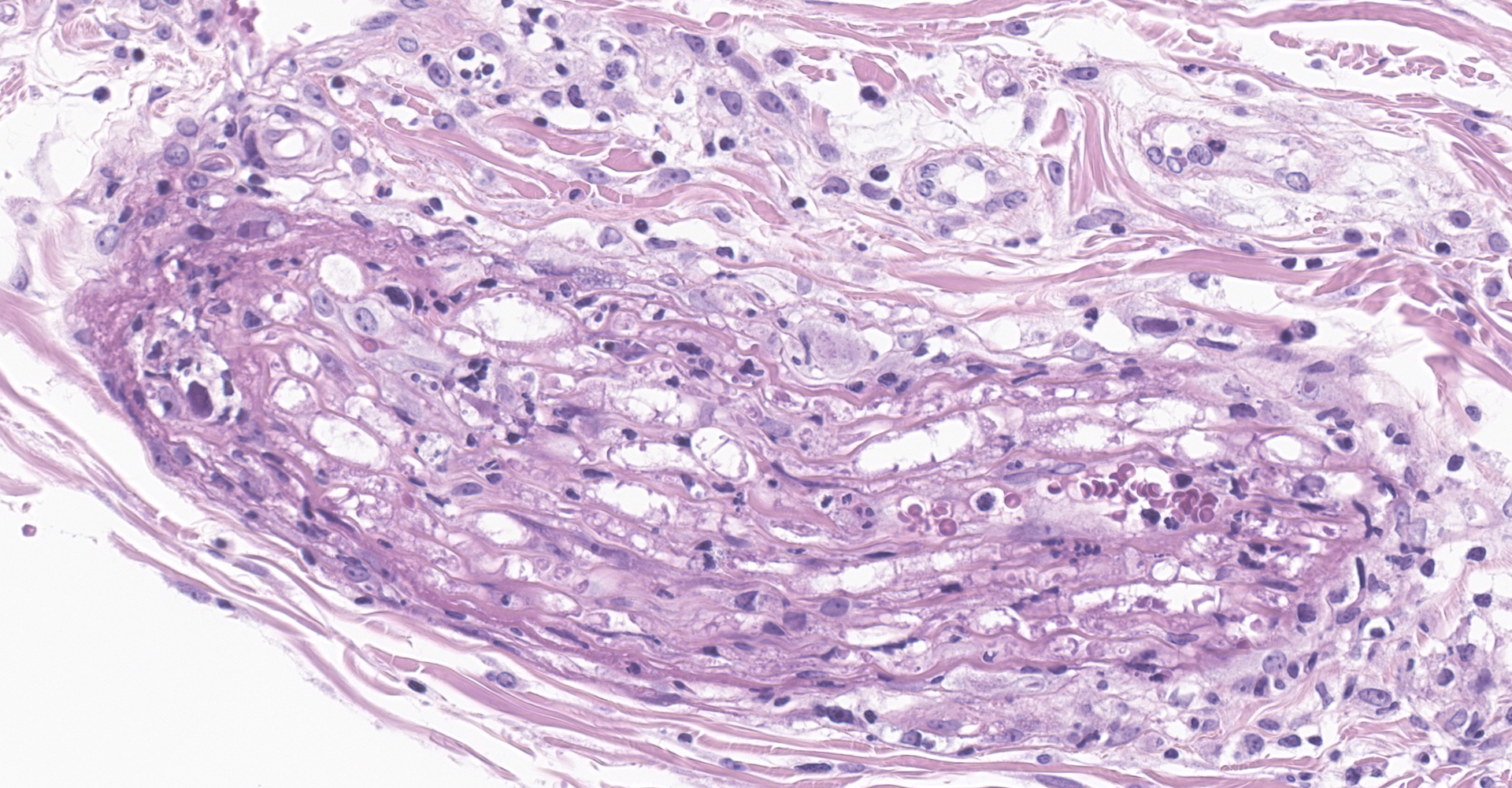
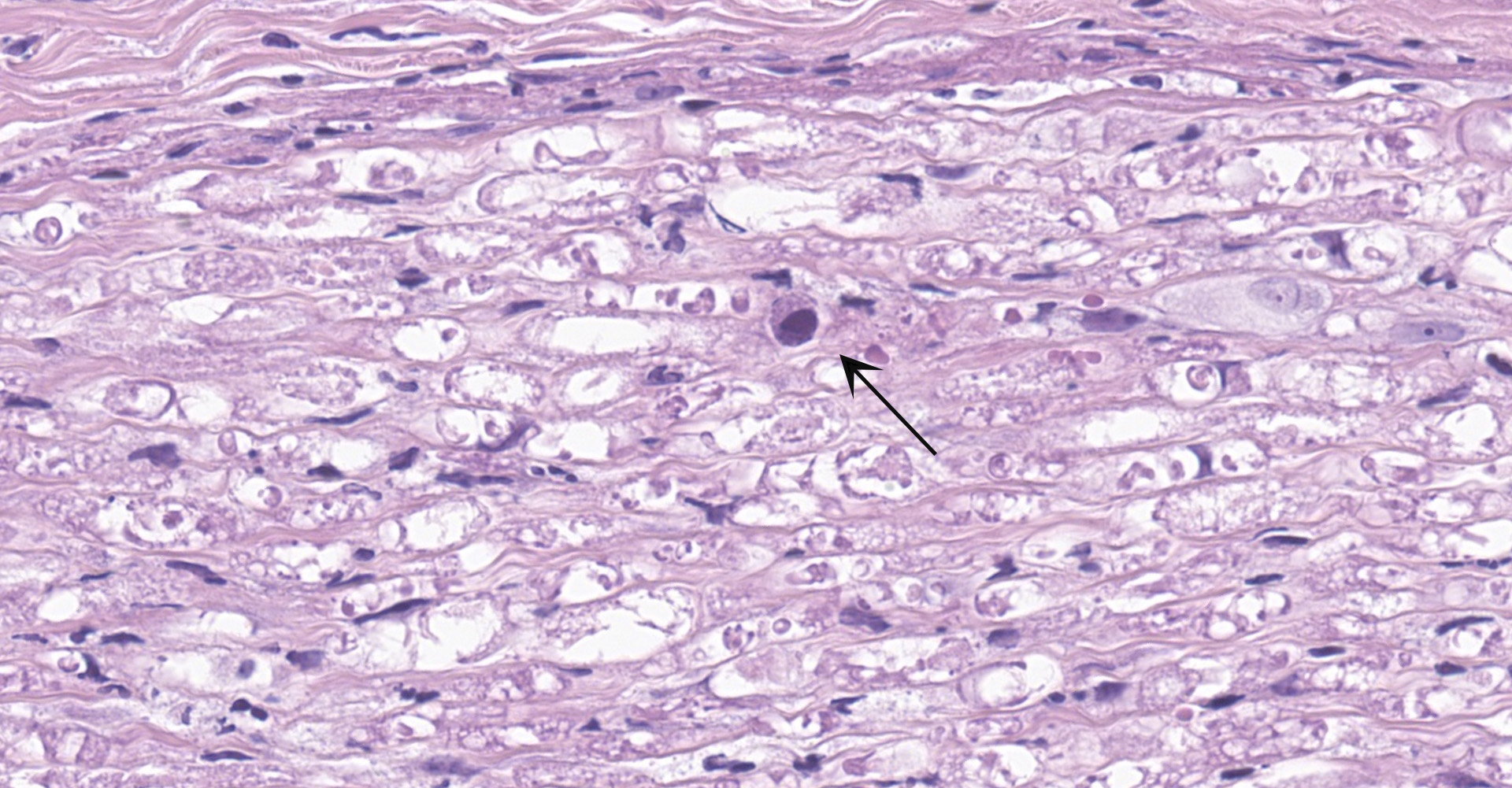
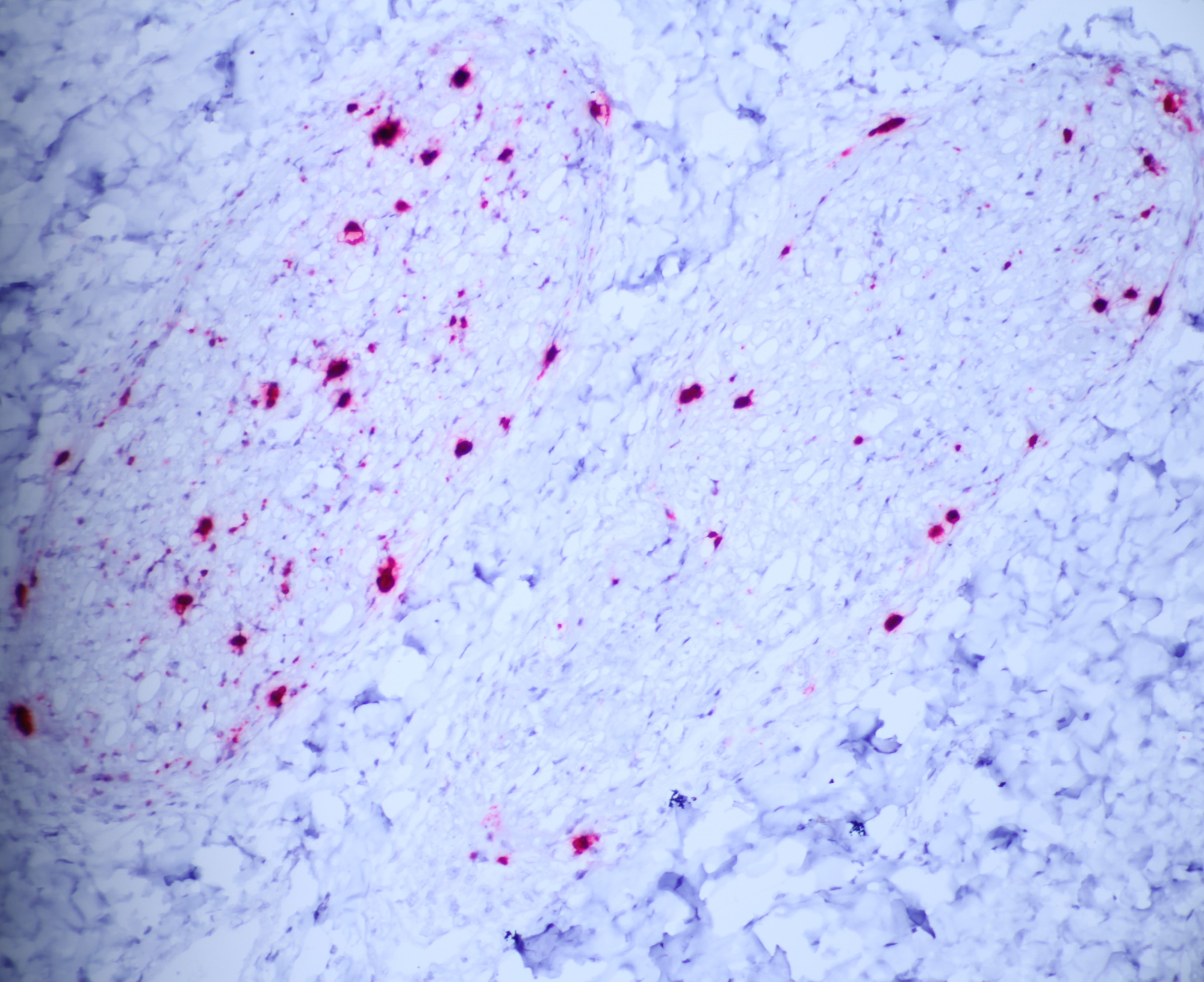
Salivary gland and soft tissues of the face ? Infiltrating the perineurium of a facial nerve are moderate numbers of neutrophils with lesser numbers of macrophages and lymphocytes. The perineurium is mild to moderately expanded by edema. Inflammatory cells (presumed macrophages) multifocally exhibit karyomegaly with intranuclear 1x3 µm, eosinophilic, hyaline inclusions that peripherally marginate the chromatin. There is marked axonal degeneration characterized by dilation of myelin sheaths with spheroid formation. Occasionally dilated axon sheaths are infiltrated by macrophages (digestion chamber formation). The surrounding fascia is similarly expanded by inflammatory cells and edema.
Contributor's morphologic diagnosis:
Nerve of the face: Neuritis, necrosuppurative, moderate, acute with axonal degeneration and intranuclear inclusions consistent with cytomegalovirus.
Contributor's comment:
Rhesus cytomegalovirus (RhCMV) is ubiquitous with a seroprevalence in rhesus macaques that nears 100% by 1 year of age.11 Experimental infection has shown that immunocompetent hosts clear virus from circulation within two weeks following the production of anti-CMV antibodies.7 Once cleared, the virus becomes latent within ganglia and animals remain infected for life. Immunosuppression, most often in AIDS and transplant recipients, results in reactivation with multiorgan pathology including interstitial pneumonia, encephalitis, enteritis, lymphadenitis, and orchitis.3 RhCMV is the most common opportunistic infection of rhesus macaques infected with SIV. Other common opportunistic infections include adenovirus, Pneumocystis sp., and Cryptosporidia sp. Facial neuritis, as seen in this case, is an uncommon presentation of RhCMV occurring in <10% of cases.1
Contributing Institution:
Division of Comparative Pathology
Tulane National Primate Research Center
https://www2.tulane.edu/tnprc/
JPC diagnosis:
Peripheral nerves: Neuritis, necrotizing, diffuse, marked with cytomegalic intranuclear inclusions.
JPC comment:
The subfamily Betaherpesvirinae encompasses four genera, which include Cytomegalovirus, Muromegalovirus, Proboscivirus, and Roseolovirus. These viruses replicate more slowly than alphaherpesviruses, leading to cytomegalic cells. During latency, these viruses are confined to hematopoietic stem cells, and possibly the cells of secretory glands and the kidney. Species within Betaherpesvirinae affect humans and non-human primates (this entity, macacine herpesvirus 3), but also infect mice (murid herpesvirus 1 and 2), rats (murid herpesvirus 8), guinea pigs (caviid herpesvirus 2), elephants (elephantid herpesviruses), and pigs (suid herpesvirus 2).8 Interestingly, the genome of macacine herpesvirus 3 encodes proteins that help evade the immune system. Peptides are produced that downregulate MHC I expression, and a viral homolog of IL-10 is produced that has anti-inflammatory properties.12 Human cytomegalovirus also produces proteins with CXC chemokine properties, encoded on viral genes UL146 and UL147. The protein encoded by UL146 is named viral CXC chemokine-1 (vCXCL1) and is at least as potent as innate IL-8 and responsible, in large part, for the neutrophilic response to this virus.10
Viral infection continues to be a relevant concern for colonies of macaques used in biomedical research. A seroprevalence study conducted in Germany of 231 macaques found high seroprevalence of cytomegalovirus (98.3%), lymphocryptovirus (89.6%), rhesus rhadinovirus (84.4%), and simian foamy virus (94.8%). These infections appear to be acquired early in life, usually before capture, but researchers should be aware of the background lesions that may be encountered during postmortem and histologic examination.6
Recent research has focused on vaccine development using cytomegalovirus as a vector. Promising vaccines have been produced against Mycobacterium tuberculosis,4,5 as well as HIV2 and Ebolavirus.9 The cytomegalovirus-vector help producing long lasting memory
lymphocytes, contributing significantly to longstanding resistance to infection.
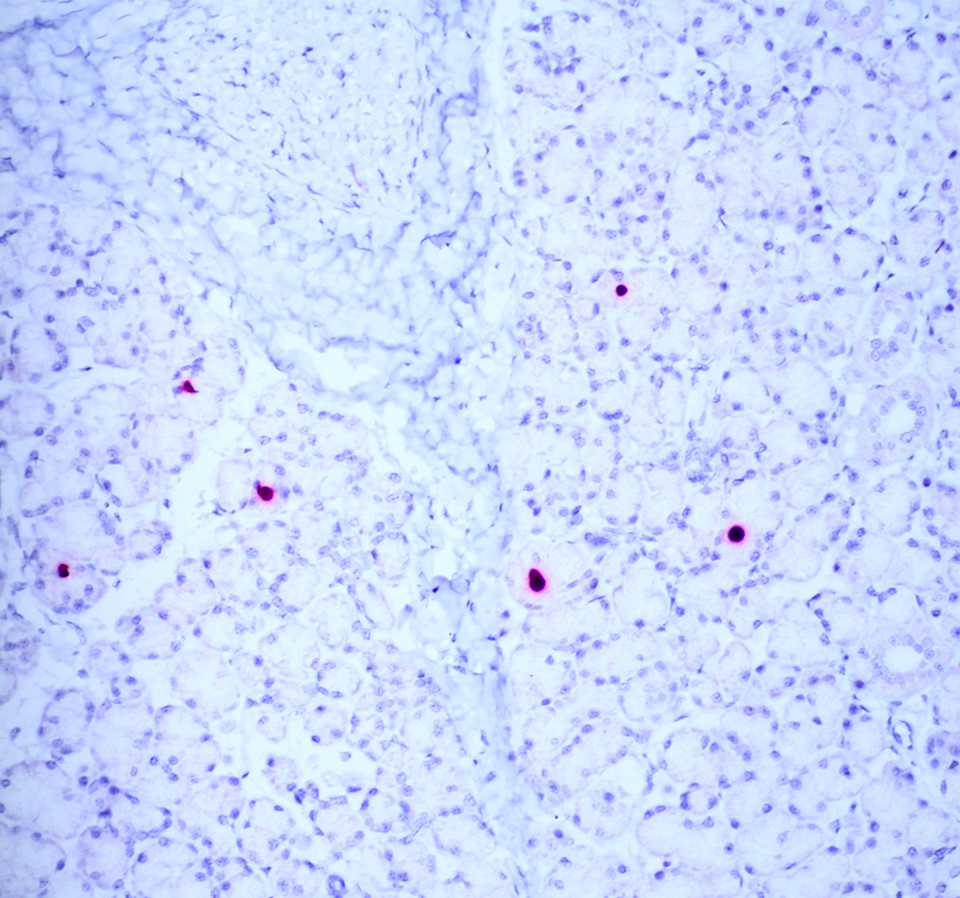
The moderator performed additional diagnostics on this case and demonstrated via in situ hybridization that affected peripheral nerves had large amounts of virus. Additionally, virus was detectable within few salivary gland nuclei, indicating a location for latent viral infection in this animal.
References:
1. Assaf BT, Knight HL, Miller AD. rhesus cytomegalovirus (macacine herpesvirus 3)-associated facial neuritis in simian immunodeficiency virus-infected rhesus macaques (Macaca mulatta). Vet Pathol. 2015;52: 217-223.
2. Barry PA, Deere JD, Yue Y, et al. Cytomegalovirus (CMV)-vectored vaccines for HIV and other pathogens. AIDS. 2020;34(3):335-349.
3. Baskin GB. Disseminated cytomegalovirus infection in immunodeficient rhesus monkeys. The American journal of pathology. 1987;129: 345.
4. Carpenter SM, Behar SM. A new vaccine for tuberculosis in rhesus macaques. Nature Medicine. 2018;24(2):124-126.
5. Hansen SG, Zak DE, Xu G, et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat Med. 2018;24(2):130-143.
6. Kaul A, Schonmann U, Pohlmann S. Seroprevalence of viral infections in captive rhesus and cynomolgus macaques. Primate Biol. 2019;6:1-6.
7. Lockridge KM, Sequar G, Zhou SS, Yue Y, Mandell CP, Barry PA. Pathogenesis of experimental rhesus cytomegalovirus infection. Journal of virology. 1999;73: 9576-9583.
8. Maclachlan NJ, Dubovi EJ, eds. Fenner's Veterinary Virology, 5th Ed. San Diego, CA:Elsevier. 2017:208-209.
9. Marzi A, Murphy AA, Feldmann F, et al. Cytomegalovirus-based vaccine expressing Ebola virus glycoprotein protects nonhuman primates from Ebola virus infection. Scientific Reports. 2016;6:21674.
10. Saederup N, Mocarski ES. Fatal attraction: cytomegalovirus-encoded chemokine homologs. Curr Top Microbiol Immunol. 2002;269:235-256.
11. Vogel P, Weigler BJ, Kerr H, Hendrickx AG, Barry PA.Seroepidemiologic studies of cytomegalovirus infection in a breeding population of Rhesus macaques. Laboratory Animal Science. 1994;44(1):25-30.
12. Wachtman L, Mansfield K. Viral Diseases of Nonhuman Primates. In: Abee CR, Mansfield K, Tardif S, Morris T, eds. Nonhuman Primates in Biomedical Research, Vol 2: Diseases, 2nd Ed. San Diego, CA:Elsevier. 2012:19-20.