WSC 22-23
Conference 3
CASE III:
Signalment:
2 year old, female intact, Domestic Shorthair cat (Felis catus)
History:
This cat presented to the primary veterinarian following acute decline at home over the previous 24 hours, characterized by acute inappetence, lethargy, labored breathing, an enlarged left submandibular lymph node, abnormal urination and defecation, and hiding behavior. The cat had been acting normally prior to the decline and no vomiting, diarrhea, coughing or sneezing were noted. The cat was indoor only and had been previously vaccinated but was not up to date. It was living with three more cats, none of whom displayed abnormal clinical signs. On physical examination, the cat was lethargic, bradycardic (130 beats/min), and tachypneic (90 breaths/min) with an audible wheeze and increased abdominal effort. The left submandibular lymph node was markedly enlarged to approximately 2 cm in diameter, while the remaining peripheral lymph nodes palpated normal. The cat was afebrile and had no obvious oral lesions.
A complete blood count (CBC) and blood smear revealed a marked leukopenia, driven by marked neutropenia and lymphopenia. FeLV and FIV testing was negative. Thoracic radiographs revealed multiple nodular soft tissue opacities throughout the lung fields, ranging in size from 0.2 cm to greater than 1.0 cm. Advanced pulmonary metastatic disease was suspected, with bacterial or fungal granuloma considered less likely.
The cat was euthanized due to the need for aggressive intervention and a guarded prognosis.
Gross Pathology:
Affecting approximately 60-70% of the total surface area of the lungs were numerous, occasionally coalescing, well demarcated, raised, slightly firm, irregularly circular, beige to dark brown masses surrounded by a thin beige rim. The masses ranged from 0.4 cm to 1.9 cm in diameter, extended deep into the lung parenchyma, and had a bulging, granular appearance on cut surface. Overall, the lungs were mottled red to dark red, wet, heavy, and oozed a small to moderate amount of red, clear, watery fluid and a small amount of pink-tinged froth on cut section. There were multiple, friable, weak adhesions between the lung lobes and the adjacent mediastinum and costal pleura. Sections of lung containing the irregularly circular nodules sank in formalin.
Laboratory Results:
Special stains:
Gram stain: The coccobacilli were Gram negative.
Bacteriology: Aerobic culture of the lungs yielded Neisseria sp. (predominant, 3+) and Pasteurella sp. (mixed, 2+) based on MALDI-TOF analysis.
Microscopic Description:
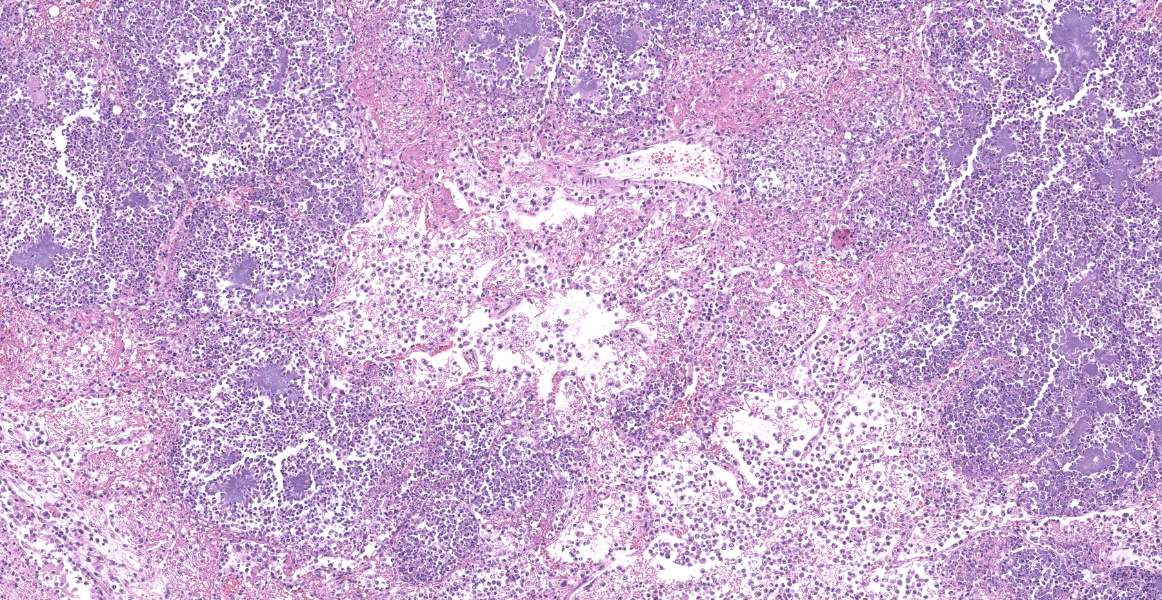
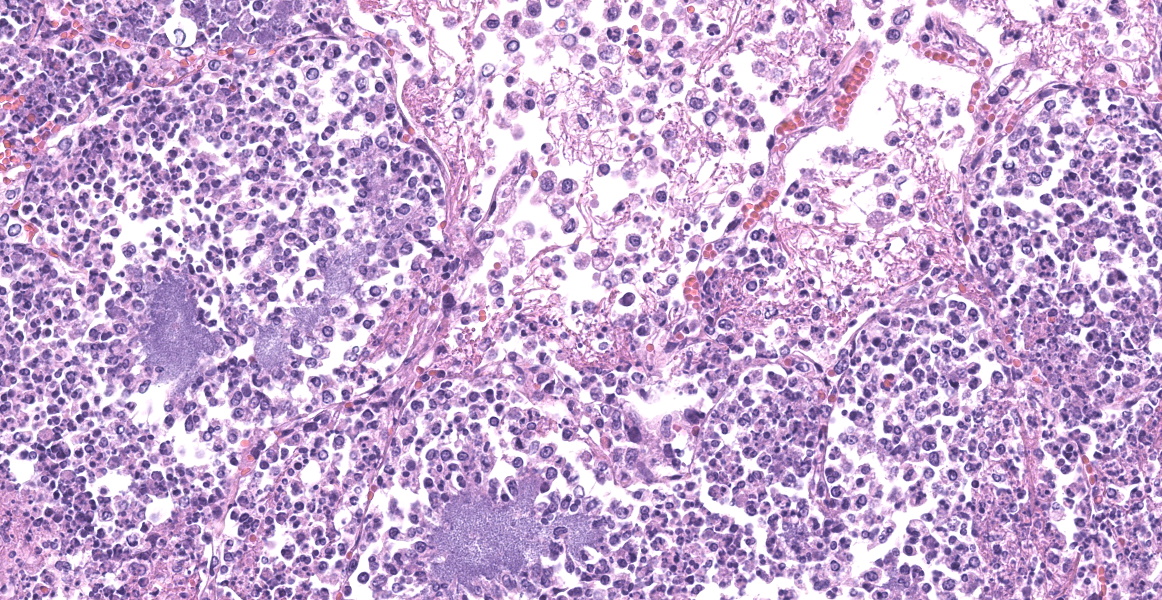
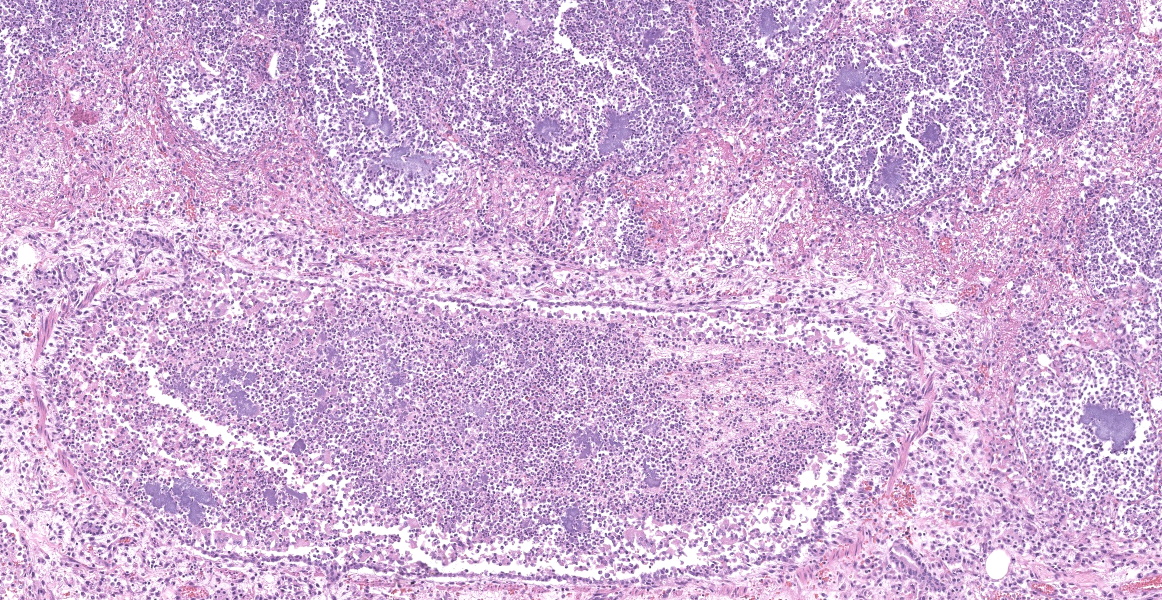
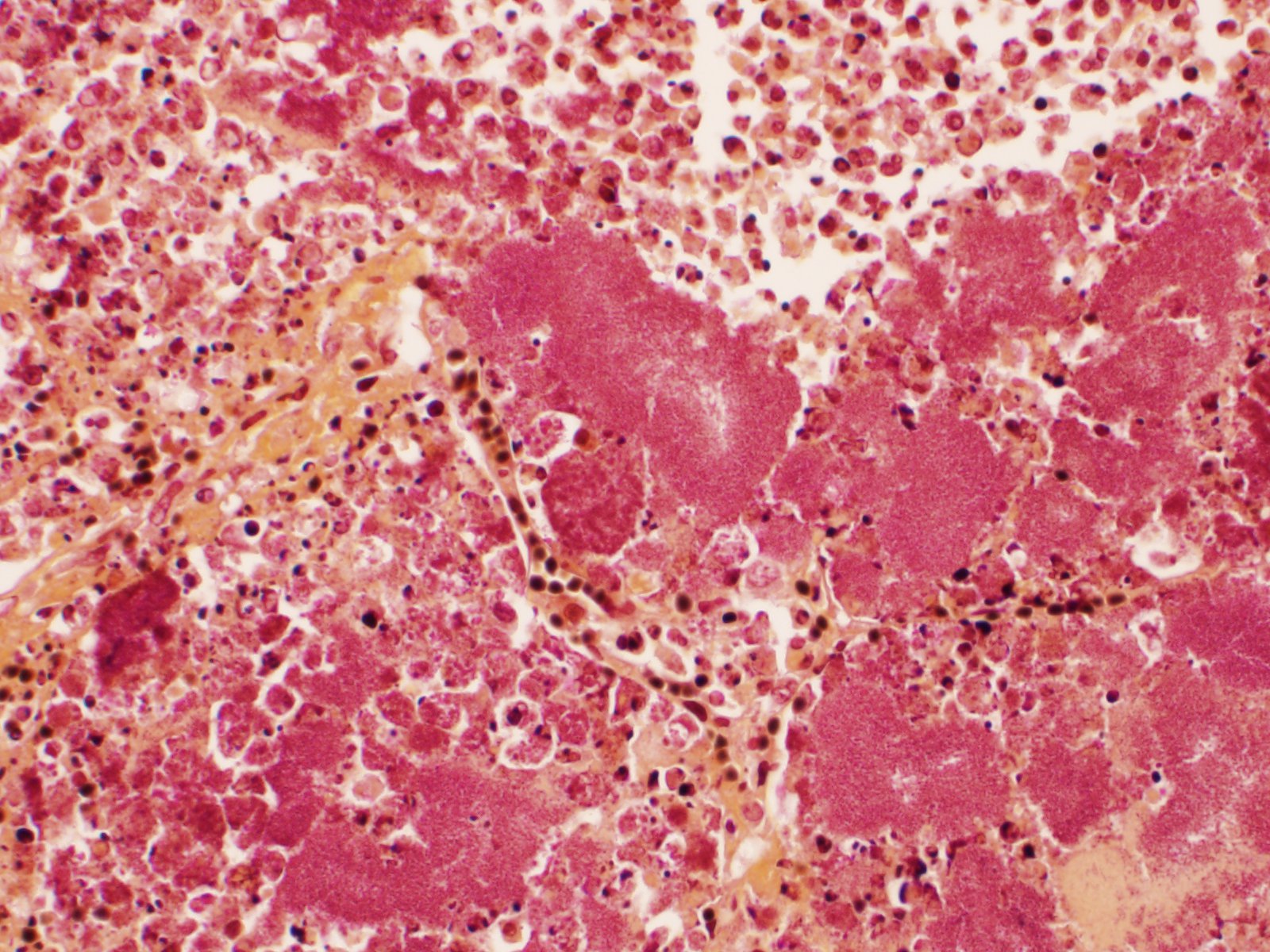
Slide B: Lungs: Widely-disseminated throughout all representative sections, affecting approximately 60-80% of the parenchyma, there are numerous, variably well demarcated nodules characterized by marked infiltration of the alveolar lumina, airways, and interstitium by degenerate and non-degenerate neutrophils admixed with abundant fibrin and large numbers of foamy macrophages. Within affected areas, alveolar septa are pale and indistinct to completely lost and replaced by large amounts of fibrin and proteinaceous and karyorrhectic debris (coagulative to lytic necrosis). Scattered bronchioles exhibit similar coagulative to lytic necrosis, with widespread sloughing and/or absence of bronchiolar epithelium. Numerous, large clusters of extracellular and occasionally intra-neutrophilic and intra-histiocytic, basophilic, approximately 1um, coccobacilli are scattered throughout areas of inflammation and necrosis. Moderate to large numbers of extravasated erythrocytes are present in the alveolar lumina, airways, and interstitium multifocally (hemorrhage). Bronchiolar lumina are variably filled by large numbers of degenerate and non-degenerate neutrophils and macrophages, moderate numbers of sloughed epithelial cells, moderate numbers of coccobacilli, and large amounts of fibrin and cytoplasmic and nuclear debris. Blood vessels within nodules occasionally contain partially-occlusive to fully-occlusive thrombi with admixed degenerate and non-degenerate neutrophils and rare bacteria. The alveolar lumina between the nodules are filled by homogenous, eosinophilic material (edema) and contain small to moderate numbers of neutrophils and macrophages. Multifocally, mesothelial cells are plump (reactive) and the pleura is mildly to moderately thickened by small to moderate numbers of neutrophils and macrophages, and small amounts of fibrin and cellular debris.
Contributor's Morphologic Diagnosis:
Lungs:
- a. Pneumonia, necro-suppurative and histiocytic, multifocal, marked, acute with intralesional, intraneutrophilic, and intrahistiocytic bacteria, bronchitis, pleuritis, hemorrhage, and bacterial emboli.
- b. Alveolar edema, diffuse, marked, acute
Contributor's Comment:
Lower respiratory tract infection is an uncommon cause of morbidity and mortality in cats. Lower respiratory tract infections are often difficult to diagnose clinically due to non-specific or inconsistent clinical, hematologic, and radiographic findings and concomitant respiratory or systemic pathology.6 Reported infectious causes associated with feline pneumonia include bacteria, viruses, fungi, protozoa, rickettsia, and parasites, with Bordetella bronchiseptica, Pasteurella sp.,Mycoplasma sp., Streptococcus sp., and Escherichia coli considered the most common bacterial agents. 6
Neisseria sp. bacteria are small, gram negative, coccobacilli, ecologically similar to Pasteurella sp. In human medicine, they are known as the causative agents of the sexually transmitted infection gonorrhea (N. gonorrhoeae), a form of bacterial meningitis (N. meningitidis), and uncommonly seen as secondary infection following feline and canine bite wounds (N. animaloris and N. zoodegmatis).7,9Neisseria sp. have been isolated as commensal flora from the oral cavity of healthy cats and dogs but are also a seldom reported cause of feline pneumonia.6,7,9 Feline Neisseria sp. pneumonia was first described in 3 domestic cats in 1973, and sporadically thereafter in cases involving further domestic cats, a tiger cub, a lion, and Chinese leopard cats.5,8,10,11
Cases of feline Neisseria sp. pneumonia are most commonly reported in adult cats, who present with variable clinical signs including acute depression, dehydration, respiratory distress, salivation, or sudden death.3,6,8,10,11 Antemortem diagnostic testing results are also variable, with the most consistent finding being discrete, multifocal nodular densities and/or lung consolidation on thoracic radiographs.6,8 Gross lesions consist of widespread, white to tan nodules scattered throughout the lung parenchyma, while the disease is histologically characterized by marked, multifocal, necrosuppurative and histiocytic, nodular pneumonia with abundant intralesional bacteria.5,8,10,11
While relatively unique, the reported radiographic, gross, and histologic findings are not considered pathognomonic for this entity without ancillary testing. Similar radiographic patterns have been reported in neoplasia and uncommonly secondary to other infectious causes, including but not limited to Yersinia pestis, Cryptococcus neoformans, Toxoplasma gondii, Mycoplasma sp., Mycobacterium sp., and rarely other bacterial, fungal or parasitic causes.3,6 While both Neisseria sp. and Pasteurella sp. bacteria were cultured from the lung in this case, Pasteurella sp. were considered less likely to be causing the primary disease, as these bacteria are common in the oral cavity and airways in healthy adult cats, are not uncommonly reported in mixed growth from feline pneumonia, and are generally associated with a suppurative bronchopneumonia when causing opportunistic lower respiratory tract disease.2 In addition, multiple historic cases of feline nodular, necrosuppurative pneumonia, both in the literature and at our institution, have yielded pure growth of Neisseria sp. on aerobic culture.
The pathogenesis of feline Neisseria sp. pneumonia is not clear, but given the distribution and vascular bacterial emboli, is considered most likely secondary to hematogenous spread, potentially from the oral cavity.8,11 The reason for the rare and severe nature of the disease given the oral commensal nature of some Neisseria species is not understood. One proposed mechanism is that chronic infection circumvents the host immune response, leading to a period of asymptomatic bacteremia and hematogenous spread to locations that favor survival and growth, with subsequent severe disease.9 Immunosuppression has been hypothesized as a potential causative factor for pathogenesis in previous case reports, however, while this case exhibited marked neutropenia and lymphopenia, there was no evidence of other pathogenic processes leading to immunosuppression within the history, clinical, gross, or histologic findings.5
Unfortunately, prognosis is poor in Neisseria sp. pneumonia cases, with decline and death generally within hours to days of onset of clinical signs, and only a single case report of successful feline Neisseria sp. pneumonia treatment.6
Contributing Institution:
Drs. Chris Bolt and Arno Wuenschmann
Minnesota Veterinary Diagnostic Laboratory and Department of Population Medicine
University of Minnesota
1333 Gortner Ave.
St. Paul, MN 55108
www.vdl.umn.edu
JPC Diagnosis:
Lung: Pneumonia, necrotizing and fibrinosuppurative, diffuse, severe with thrombosis and large colonies of extracellular and intracellular cocci.
JPC Comment:
Neisseria animaloris and Neisseria zoodegmatus were previously known as Centers for Disease Control (CDC) Group Eugonic Fermenters (EF) 4a and 4b, respectively, until they were determined to belong to the Neisseria genus in 2006.13 As the contributor states, Neisseria sp. are commensal organisms of the oral cavity of dogs and cats, and in addition to infrequently causing pneumonia, can rarely cause subcutaneous infections of the neck and facial regions in cats. One report described a young adult Russian Blue cat with a submandibular mass characterized by necrosis, purulent inflammation, and fibrosis secondary to Neisseria animaloris (EF-4a at the time).1 In a separate report, a young adult domestic short hair cat developed subcutaneous swelling over the bridge of the nose due to pyogranulomatous inflammation and Neisseria sp infection.4 Routine stains did not illustrate bacteria in the first case; however, in the second case, Gram negative, ZN-negative coccobacilli with a PAS-positive capsule were identified within macrophages. Both cases responded to debulking and antibiotic therapy, and traumatic inoculation from penetrating injury were suspected to be the initial cause of infection.1,4
As the contributor mentioned, N. meningitidis and N. gonorrhoeae are significant pathogens in humans. While most Gram-negative bacteria use siderophores to obtain iron, pathogenic (non-commensal) Neisseria species have evolved a unique mechanism to extract iron from host serum transferrin (TF), the predominant mammalian transport protein for iron.12 The bacterial transferrin receptor consists of two parts: TF-binding protein B (TbpB, a surface lipoprotein), which initially harnesses TF, and TF-binding protein A (Tbp A, a TonB-dependent transporter), which removes and transports ferric acid into the bacteria through a conformationally-active pore.12 While the majority of research on Tbps has been conducted in Neisseria species, similar receptors have been found in Pasteurellaceae and Moraxellaceae species, including Actinobacillus pleuropneumoniae and Histophilus somni.12 Bacterial Tbp activity appears to be host-species specific and increases pathogen virulence within the host.
References:
- Baral RM, Catt MJ, Soon L, et al. Successful treatment of a localized CDC group EF-4a infection in a cat. J Feline Med Surg. 2017;9(1):67-71.
- Bart, M et al. Feline Infectious Pneumonia: A Short Literature Review and a Retrospective Immunohistological Study on the Involvement of Chlamydia spp. and Distemper Virus. The Veterinary Journal. 2000; 159:220-230.
- Carniel F et al. What Is Your Diagnosis? J Am Vet Med Assoc. 2020; 257(4):375-377.
- Carr SV, Martin PA, Keyes L, Tong LJ, Talbot JJ, Muscatello G, Barrs VR. Nasofacial infection in a cat due to a novel bacterium in Neisseriaceae. JFMS Open Rep. 2015;1(2):1-5.
- Ceyssens et al. Necrotizing Pneumonia in Cats Associated with Infection of EF-4a Bacteria. J Vet Med. 1989; 36(4):314-316.
- Foster S, Martin P. Lower Respiratory Tract Infections in Cats - Reaching beyond empirical therapy. J Feline Med Surg. 2011; 13(5):313-332.
- Heydecke A et al. Human wound infections caused by Neisseria animaloris and Neisseria zoodegmatis, former CDC Group EF-4a and EF-4b. Infection Ecology and Epidemiology. 2013; 3: 20312.
- Jang SS et al. Focal Necrotizing Pneumonia in Cats Associated with a Gram Negative Eugonic Fermenting Bacterium. Cornell Vet. 1973; 63:446-454
- Liu G et al. Non-Pathogenic Neisseria: members of an abundant, multi-habitat, diverse genus. Microbiology. 2015; 161:1297-1312
- Lloyd J, Allen JG. The isolation of Group EF-4 bacteria from a case of granulomatous pneumonia in a tiger cub. Aust Vet J. 1980; 56(8):399-400.
- Perry AW, Schlingman DW. Pneumonia Associated with Eugonic Fermenter-4 Bacteria in Two Chinese Leopard Cats. Can Vet J. 1988; 29(11): 921-922.
- Pogouste AK, Morales TF. Iron acquisition through the bacterial transferrin receptor. Crit Rev Biochem Mol Biol. 2017: 52(3):314-326.
- Vandamme P, Holmes B, Bercovier H, Coenye T. Classification of Centers for Disease Control Group Eugonic Fermeter (EF)-4a as Neisseria animaloris nov. and Neisseria zoodegmatis sp. nov, respectively. Int J Syst Evol Microbiol. 2006:56(Pt8):1801-1805.