Joint Pathology Center
Wednesday Slide Conference
2017-2018
Conference 22
April 18th, 2018
CASE I: 15L-2067C (JPC 4066542).
Signalment: 3-year-old, male, Warmblood (Equus caballus), equine.
History: Acute signs of colic, poor general condition
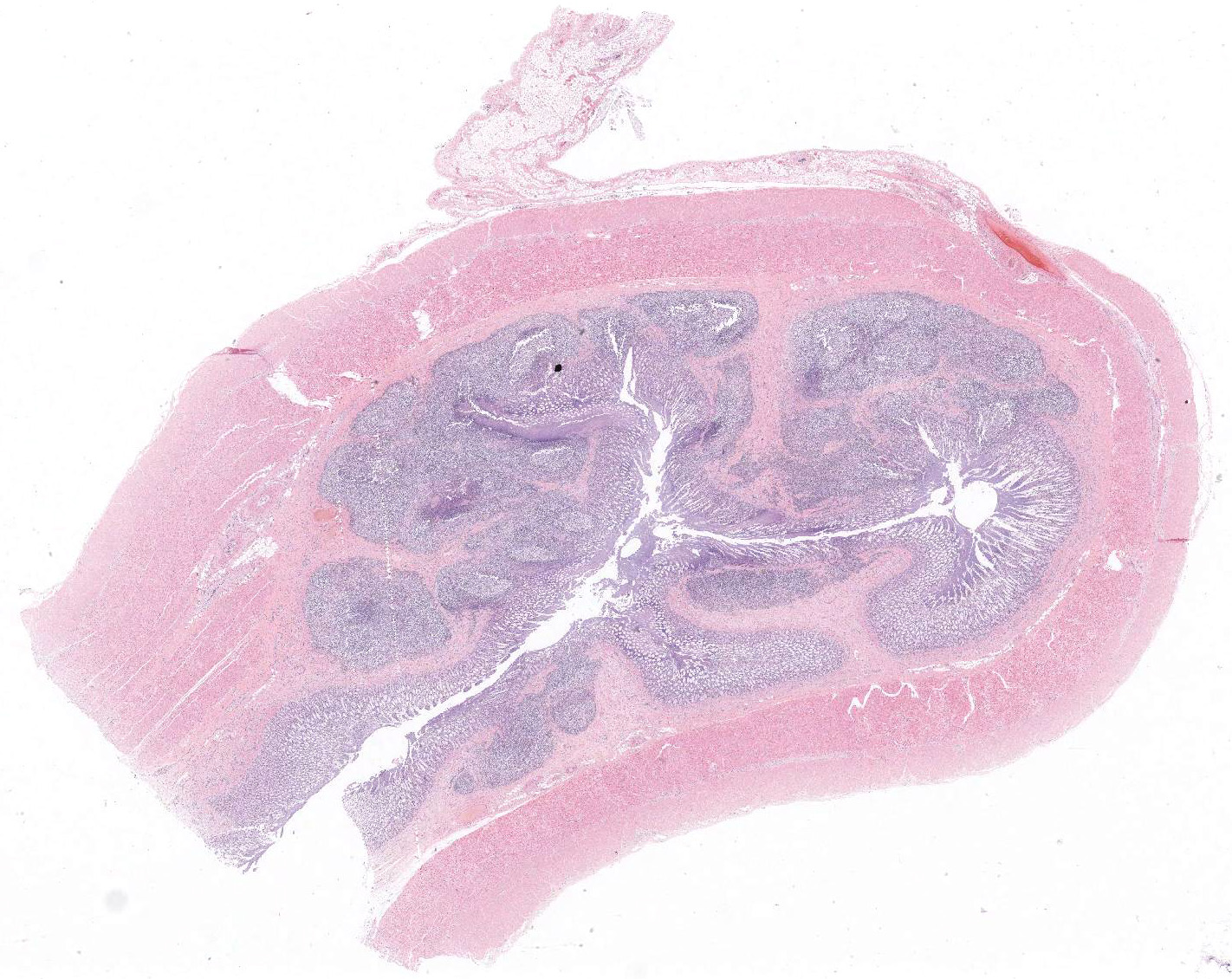
Gross Pathology: Diffuse thickening and white discoloration of the caudal 2/3 of the small intestine, evident from the serosal surface.
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.):
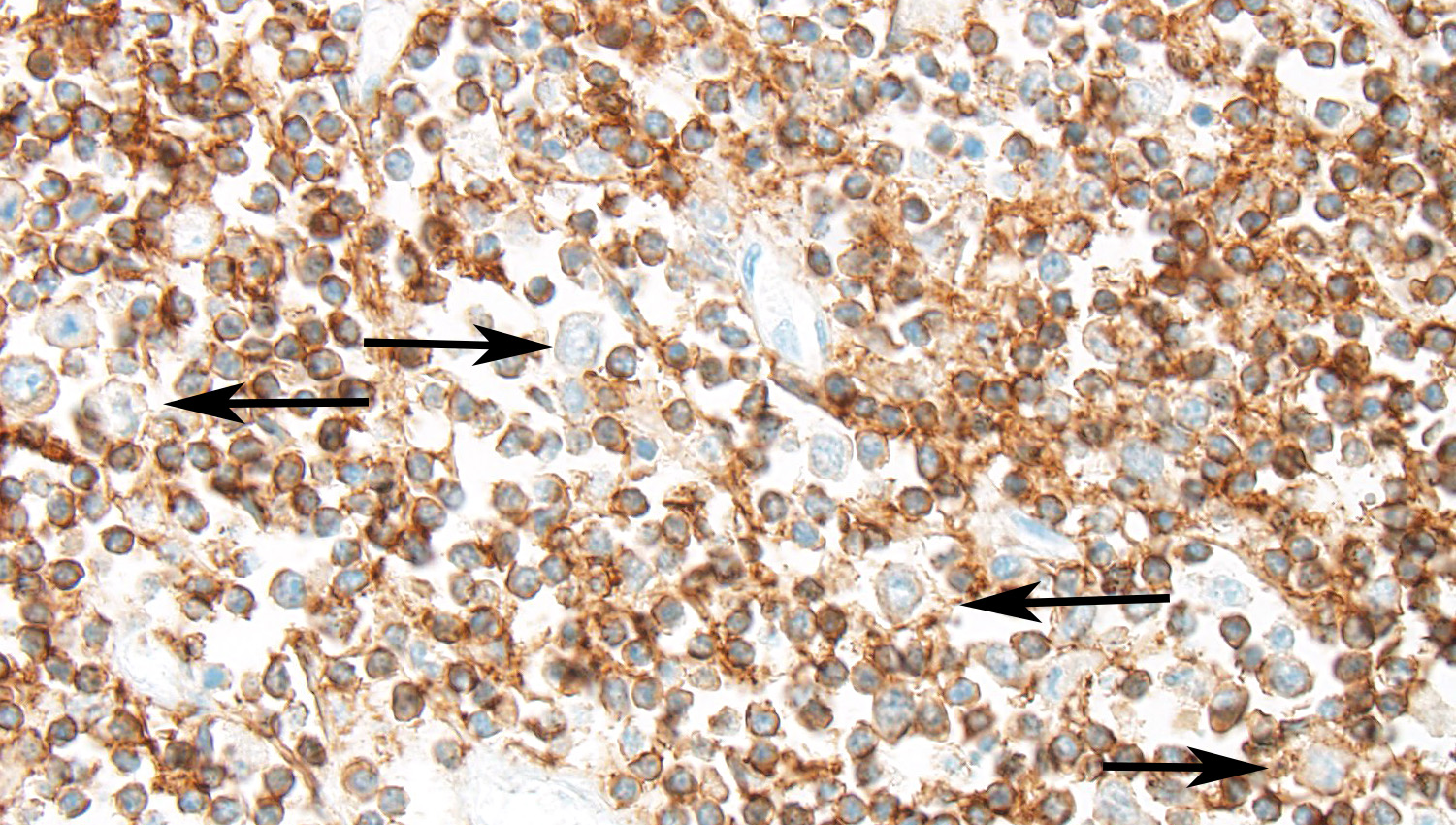
- CD3: Diffusely well-differentiated small lymphocytes show moderate to strong immunopositivity for CD3. Large blastic cells show diffuse moderate membranous positive signal for CD3. CD3 positive cells represent >95% of the lymphoid cells recognized.
- CD79a: Scattered very rare small well-differentiated lymphocytes are positive for CD79a antibody. Large blastic cells are negative. CD79a positive cells represent <5% of the lymphoid cells recognized.
- CD20: Large blastic cells are diffusely negative for CD20 antibody. CD20 positive cells represent <5% of the lymphoid cells recognized.
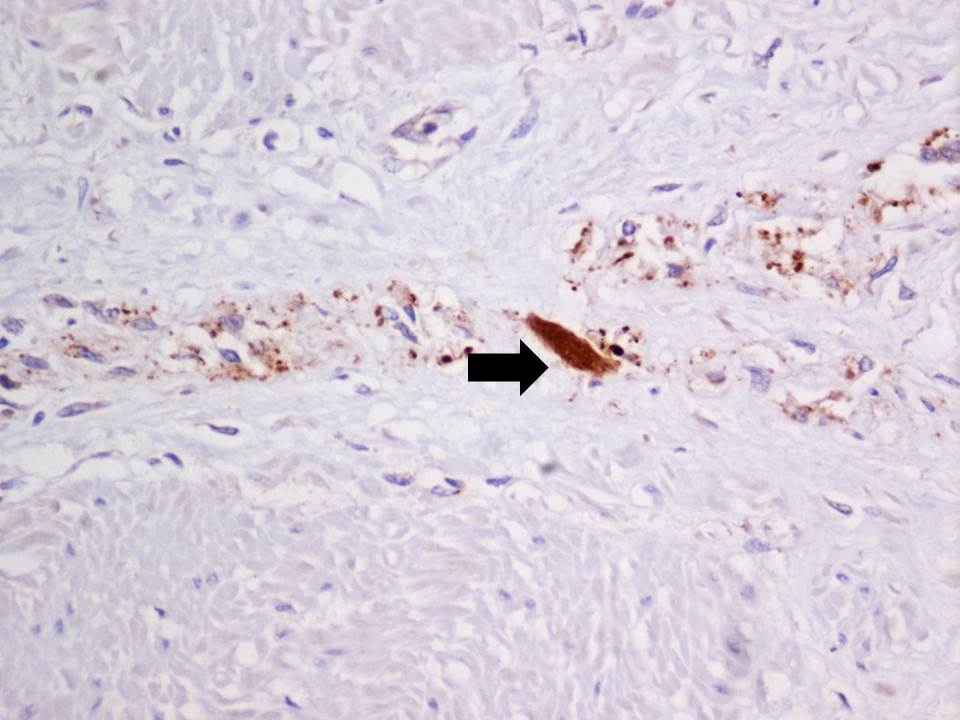
- Synaptophysin: An intense strong cytoplasmic positive stain is detected in the scattered remaining neurons of the Auerbach’s and Meissner’s plexuses
Microscopic Description:
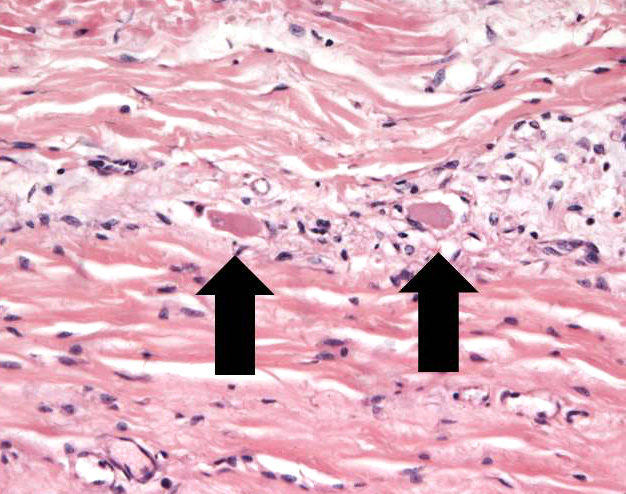
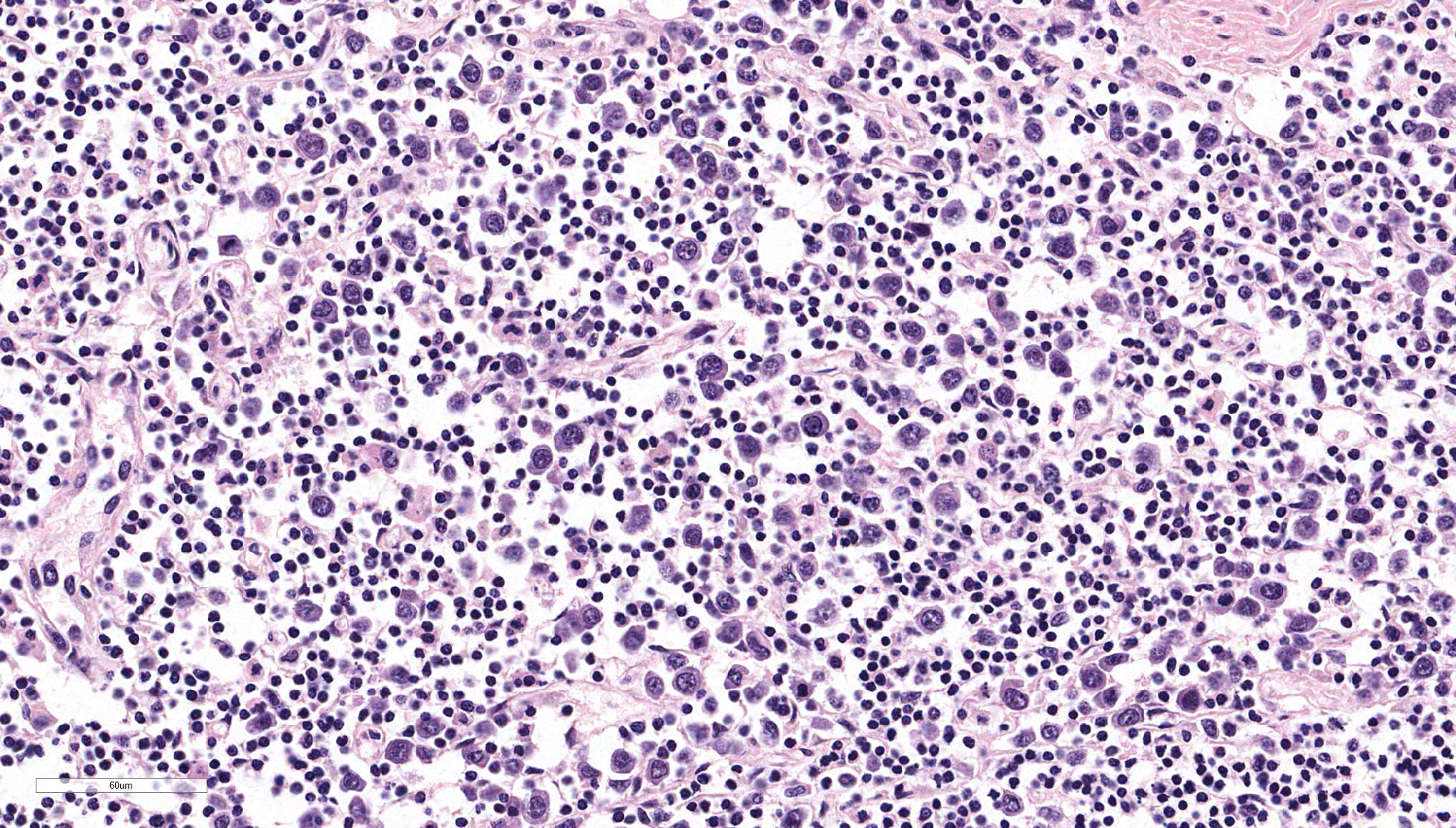
Ileum – The intestinal epithelium is diffusely effaced by amorphous eosinophilc material and nuclear debris (post mortem autolysis – artifact). Multifocally, some crypts are expanded by eosinophilic and basophilic amorphous material (cellular and nuclear debris/crypt abscesses) with occasional areas characterized by granular basophilic material (mineralization – dystrophic). The autonomic ganglia of the submucosa (Meissner’s) and tunica muscularis (Auerbach’s) exhibited a marked reduction in numbers of neuronal bodies which occasionally appear hypereosinophilic with rounded cell margins and peripheralized hyperchromatic nuclei (chromatolysis). Within the plexuses an increased number of satellite cells is also recognized. Expanding from the submucosal gut associated lymphoid tissue (GALT), and diffusely infiltrating and effacing the mucosa, submucosa and occasionally reaching the superficial tunica muscularis of the ileum there is a densely cellular, poorly demarcated, unencapsulated and infiltrative proliferation of round cells arranged in sheets within a fine fibrous stroma. Cells are 20-25 μm in diameter, round with distinct cell borders. They exhibit scant to moderate lightly basophilic cytoplasm and central round nuclei with finely stippled to vesicular chromatin and one to three nucleoli. The follicular structure of GALT is diffusely effaced or lost. Mitotic index is 1-4 mitosis per high power field and occasional bizarre mitoses are recognized. Large numbers of small well-differentiated lymphocytes are infiltrating in between the atypical blastic cells. Large numbers of atypical lymphoid cells are found infiltrating lymphatic vessels and/or veins of the lamina propria, submucosa, muscularis and serosa. Scattered basophilic, star-shaped bodies (mineralization – asteroid bodies) are recognized within the intima of medium sized to large submucosal arteries (incidental finding).
Contributor’s Morphologic Diagnosis:
Ileum, diffuse sub-acute severe Meissner’s and Auerbach’s plexuses chromatolysis and neuron loss.
Ileum, atypical lymphoproliferative disorder consistent with intestinal lymphoma.
Name of the condition: Equine dysautonomia
Name of the disease: Equine grass sickness
Contributor’s Comment: In the present case, the submucosal (Meissner’s) and myenteric (Auerbach’s) plexuses showed severe neuronal chromatolysis (degeneration) consistent with equine grass sickness (equine dysautonomia). Immunohistochemical staining with an anti-synaptophysin antibody revealed a marked reduction in numbers of neurons within the neuronal ganglia of the Meissner’s and Auerbach’s plexuses and a highly increased immune-signal within the soma of remnant neurons indicative of neuronal degeneration.3,20
Equine grass sickness is a polyneuronopathy of the central and peripheral nervous system that only affects grazing horses, ponies and donkeys. It is a seasonal disease with peak of incidence between April-July in the northern hemisphere. This pathological condition was first described in Scotland in 1909, since then equine grass sickness has been described in numerous Northern European countries, Cyprus, Falkland Islands and Australia.17 Although the etiology is still unknown, toxins produced by Clostridium spp. are suspected to play a role in the disease as low serum antibody levels for this bacterium were found to be a risk factor.10 The most common gross pathological manifestations are fluid distention of the proximal gastrointestinal tract (stomach and small intestine) and large intestine impaction with dry and corrugated digesta often coated by copious mucus.1 Histologically the main findings consist in neuronal chromatolysis of the peripheral nervous system and central nervous system, with changes particularly severe in the peripheral autonomic ganglia and enteric neurons. Whilst less significant, different studies also have identified involvement of the central nervous system including degeneration of cranial nerves (III, V, VI, VIII, XII), dorsal motor nucleus of the X, accessory cuneate nucleus, red nucleus and reticular formation.17 The histological appearance of the chromatolytic neurons consists in swelling of cell body (soma), loss and/or peripheralization of Nissl substance, central eosinophilic spheroid bodies, foamy cytoplasm and peripheral margination and flattening of the nucleus.9 The best gastrointestinal location to identify these changes is the ileum (submucosal and myenteric plexuses), especially in acute cases.13 Nonetheless, chromatolysis has been also reported in stomach, duodenum, jejunum, caecum, large colon, small colon and rectum,17 and the duodenum has been identified as the best location to identify degenerated neurons in chronic cases.13 Neuronal lesions have been documented in the following ganglia of the autonomic system: ciliary ganglion, cranial cervical ganglion, caudal mesenteric ganglion, stellate ganglion, thoracic and abdominal sympathetic trunk, celiaco-mesenteric, caudal mesenteric ganglion and parasympathetic terminal cardiac ganglion.17 Additionally, loss of interstitial cells of Cajal have also been reported in cases of acute grass sickness, suggesting the loss of these pacemarkers may also contribute to development of the dysmotility.6 The only way to diagnose equine grass sickness ante mortem is by mean of an ileal biopsy which allows the identification of neuronal loss and / or neuronal chromatolysis with 100% sensitivity and specificity.12 Anti-synaptophysin immunostaining has been proposed as a good diagnostic tool to identify degenerating neurons which show an increase of synaptophysin signal within the cytoplasm.3,20
Associated with the typical features of equine dysautonomia, an atypical proliferation of the lymphoid tissue arising from the GALT was also recognized in this case, that can explain the grossly observed focally extensive thickening and white discoloration of the intestine, which is not a usual feature of equine grass sickness per se. In our opinion there are several features that support the diagnosis of a lymphoma against a lymphoid hyperplasia: grossly the intestinal wall was diffusely severely thickened and white in color. Histologically the blastic lymphoid cells exhibited features of atypia (large cellular size, with vesicular to finely stippled chromatin, occasional nuclear membrane indentations and scattered bizarre mitotic figures) and the infiltrative behavior (invasion of the lamina propria and vessels) with loss of follicular structure of numerous GALT areas. On the basis of the morphological appearance provided by the H&E the tumor was provisionally classified as a T-cell rich B-cell lymphoma.
Immunohistochemichal results however identified a constant CD3 positivity in both small and blastic lymphoid cells, with lack of positivity for B markers (CD79a / CD20). CD3 positive cells were also detected within vessels. On this basis a T cell lymphoproliferative disorder consistent with a T cell lymphoma was the final diagnosis in this case. Unfortunately no other organ was available for examination to further investigate the possible leukemic phase of the neoplasm as suggested by the vascular invasion, in distant organs. A clonality test was not available to test the clonality of the proliferation.
Alimentary lymphoma is a commonly reported tumor of the gastrointestinal system in horse.1 The mean age of presentation of intestinal lymphoma is 16 years. This information, gained from a recent publication19, differs from older papers of equine lymphoma in which the mean age was 7.5-10 years.5,14,15 This difference may suggest alimentary lymphoma is more represented in older animals compared to other lymphomas or perhaps life expectancy of horses has increased during recent years.19 The major clinical signs are weight loss, ventral edema and ascites probably due malabsorption and protein loss, lethargy, occasionally diarrhea, pyrexia, and abdominal pain with sings of colic.1,5 In one study lymphoma was found as the most common diagnosis in a group of horses showing frequent recurrent episodes of colic with high mortality, lymphoma was the fourth most common etiology in horses showing chronic colic signs.4,8 The most affected anatomical region is the small intestine closely followed by the large intestine, although segmental distribution is also described usually associated with younger animals.19 The gross appearance of this tumor consists of diffuse thickening of the intestinal wall, enlargement of the regional lymph nodes and sometimes nodular bulges in the serosal surface.1,5,18 Histologically it is commonly characterized by diffuse infiltration of the mucosa and submucosa and sometimes the tunica muscularis.1 The cytological features of the tumor may vary depending on phenotype of the primary neoplastic lymphocytes.7,11
Alimentary lymphoma has been traditionally correlated with B-cell neoplastic proliferation since it is thought to arise from GALT, therefore large centroblastic cells are the predominant cell population.1,18 Other subtypes of lymphoma have been later documented such as epitheliotrophic T-cell lymphoma16 and T-cell rich B-cell lymphoma (TCRBCL), been this latter the most common lymphoma subtype according to a recent publication.2 Histologically TCRBCL is composed of two cell populations, the predominant one is characterized by well-differentiated small T-lymphocytes, intermingled within these T-cells there are large neoplastic cells ~2-3 times the size of the aforementioned cells which are derived from B-lymphocytes.2,7 Most of T-cell lymphomas are characterized by small to medium T-lymphocyte cell population infiltrating lamina propria and mucosa occasionally showing epitheliotropsim.16 In all lymphomas there is a marked shortening and fusion of the villi.
JPC Diagnosis: 1. Small intestine (ileum per contributor): Lymphoma (consistent with TCRBCL), Warm blood (Equus caballus), equine.
- Small intestine (ileum per contributor), neurons: Neuronal degeneration and loss, multifocal, moderate.
Conference Comment: Gastrointestinal lymphomas are discussed by the contributor and were reviewed by conference attendees including: enteropathy-associated T-cell lymphomas, type I and II (EATL), diffuse large B cell lymphomas, and large granular lymphocyte (LGL) lymphoma. EATL is most common in the jejunum of dogs and cats. EATL type I is composed of intermediate to large T-cells, whereas EATL type II is composed of small T-cells (often with histologic overlap with inflammatory bowel disease early in disease process).LGL lymphoma is composed of large cells with brightly eosinophilic granules that contain granzymes. The granules are much easier to see cytologically, so an impression smear done by the surgeon prior to formalin fixation is often high yield.
In this case, there are large cells (that are often mitotic) admixed with a reactive population of small lymphocytes. In the horse, this mixed cell population is most consistent with T cell rich large B cell lymphoma (TCRBCL), which is the most common subtype of lymphoma in this species. For this reason, we re-ran special stains to take a second look at the two cell populations. CD3, a marker for T-cells, was positive (with strong intracytoplasmic immunoreactivity) for the majority of the cells, particularly the cells that expand the lamina propria; CD20 and Pax-5, markers for B-cells were positive (with strong intracytoplasmic immunoreactivity) for the smaller population of large neoplastic cells admixed among the T-cell aggregates or forming germinal centers (presumed to be residual GALT) or was non-contributory, respectively. It is ideal to use more than one B-cell marker in suspected lymphoma cases. CD79a was not run in this case as it has not been proven effective in the horse. Two other B-cell markers (CD20 and Pax-5) This immunohistochemical pattern is compatible of TCRBCL. PARR was discussed as an option for diagnosis, but the paucity of neoplastic cells and the abundance of small reactive T cells may confound this test.
Contributing Institution:
University of Liverpool,
Leahurst Campus,
Chester High Road,
Neston, Wirral, UK, CH64 7TE
References:
- Brown CC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, 5th ed.Vol 2. Philadelphia, PA:Elsevier Saunders;2007:1-296.
- Durham AC, Pillitteri CA, San Myint M, et al. Two hundred three cases of equine lymphoma classified according to the World Health Organization (WHO) classification criteria. Vet Pathol. 2013;50:86-93.
- Hilbe M, Guscetti F, Wunderlin S, et al. Synaptophysin: an immunohistochemical marker for animal dysautonomias. J Comp Pathol. 2005;132:223-227.
- Hillyer MH, Mair TS. Recurrent colic in the mature horse: a retrospective review of 58 cases. Equine Vet J. 1997;29:421-424.
- Hillyer TSMaMH. Clinical features of lymphosarcoma in the horse: 77 cases. Equine vet Educ. 1991;4:108-113.
- Hudson N, Mayhew I, Pearson G. A reduction in interstitial cells of Cajal in horses with equine dysautonomia (grass sickness). Auton Neurosci. 2001;92:37-44.
- Kelley LC, Mahaffey EA. Equine malignant lymphomas: morphologic and immunohistochemical classification. Vet Pathol. 1998;35:241-252.
- Mair TS, Hillyer MH. Chronic colic in the mature horse: a retrospective review of 106 cases. Equine Vet J. 1997;29:415-420.
- Vandevelde RJH, Oevermann A. Veterinary Neuropathology: Essentials of Theory and Practice. New York, NY:Wiley-Blackwell; 2012:15-16.
- McCarthy HE, French NP, Edwards GB, et al. Equine grass sickness is associated with low antibody levels to Clostridium botulinum: a matched case-control study. Equine Vet J. 2004;36:123-129.
- Meyer J, Delay J, Bienzle D. Clinical, laboratory, and histopathologic features of equine lymphoma. Vet Pathol. 2006;43:914-924.
- Milne EM, Pirie RS, McGorum BC, et al. Evaluation of formalin-fixed ileum as the optimum method to diagnose equine dysautonomia (grass sickness) in simulated intestinal biopsies. J Vet Diagn Invest. 2010;22:248-252.
- Murray A, Pearson GT, Cottrell DF. Light microscopy of the enteric nervous system of horses with or without equine dysautonomia (grass sickness): its correlation with the motor effects of physostigmine. Vet Res Commun. 1997;21:507-520.
- Neufeld JL. Lymphosarcoma in the horse: a review. Can Vet J. 1973;14:129-135.
- Neufeld JL. Lymphosarcoma in a mare and review of cases at the Ontario Veterinary College. Can Vet J. 1973;14:149-153.
- Pinkerton ME, Bailey KL, Thomas KK, et al. Primary epitheliotropic intestinal T-cell lymphoma in a horse. J Vet Diagn Invest. 2002;14:150-152.
- Pirie RS, Jago RC, Hudson NP: Equine grass sickness. Equine Vet J. 2014;46:545-553.
- Platt H: Alimentary lymphomas in the horse. J Comp Pathol. 1987;97:1-10.
- Taylor SD, Pusterla N, Vaughan B, et al. Intestinal neoplasia in horses. J Vet Intern Med. 2006;20:1429-1436.
- Waggett BE, McGorum BC, Shaw DJ, et al. Evaluation of synaptophysin as an immunohistochemical marker for equine grass sickness. J Comp Pathol. 2010;142:284-290.