Signalment:
Adult female C57BL/6 mouse (
Mus musculus).This mouse was treated with six doses of 7,12-dimethylbenz[a]anthracene (DMBA) at 10mg/ml to induce mammary carcinomas.
Gross Description:
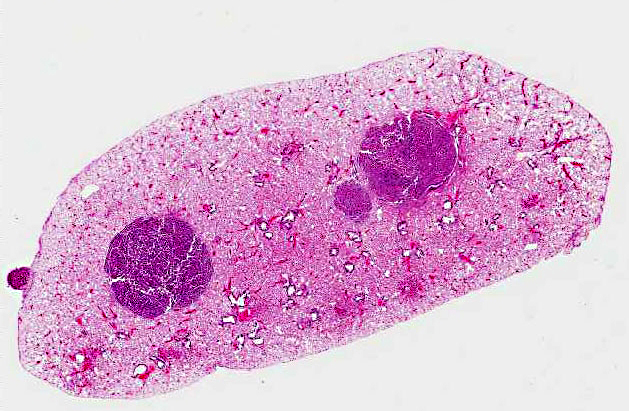
On gross examination, there was an irregular, expansile, 13x18x18mm mass on the seventh and eighth mammary glands within the right axillary region. The mass was pale tan on cut surface with multifocal 2-4mm foci of hemorrhage and necrosis. Internally, the lungs were multifocally mottled pink to dark red, and there were two 1-2mm white foci on the right caudal lung lobe, and two 3-4mm white foci on the left lung lobe.
Histopathologic Description:
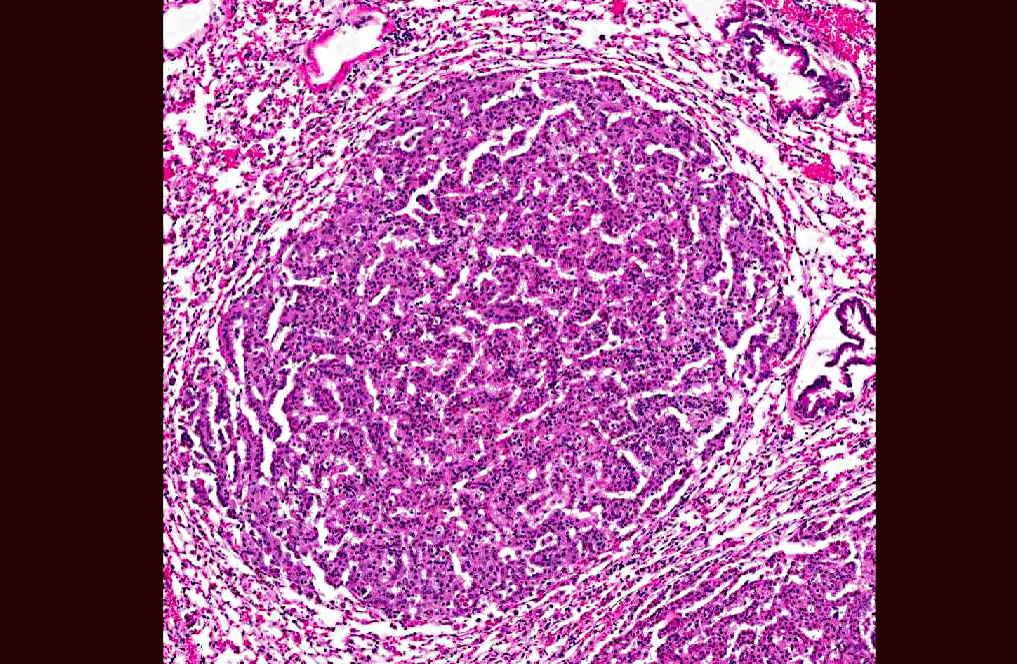
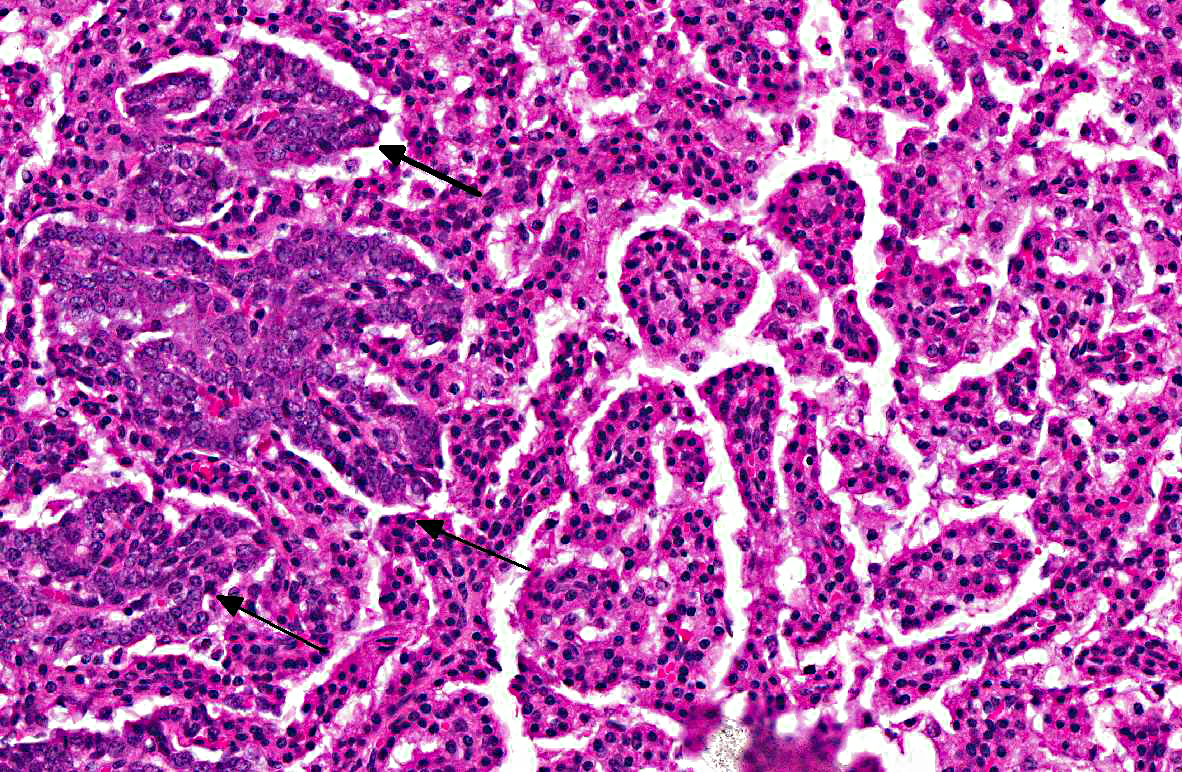
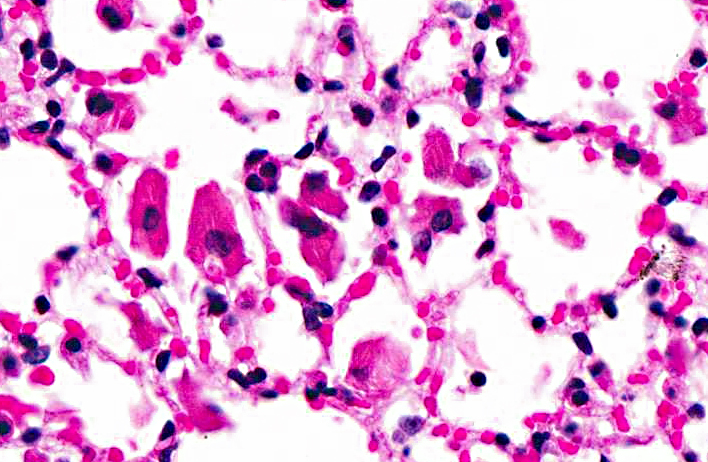
Lung: In sections of lung, there were one to multiple, expansile, well-demarcated, unencapsulated proliferations of well-differentiated epithelial cells compressing the adjacent pulmonary architecture and collapsing subjacent alveolar spaces. The masses were composed of lobules, cords, and papillary projections of cuboidal to columnar epithelial cells with moderate faintly eosinophilic amorphous cytoplasm and a central round nucleus with coarsely clumped to condensed chromatin and an occasional nucleolus, supported by a fine fibrovascular stroma. Mitotic figures were not observed. Adjacent to and within the surrounding alveolar spaces were small to large numbers of large macrophages with moderate to abundant brightly eosinophilic cytoplasm containing refractile needle-shaped to acicular crystalline material. Occasional to frequent multinucleate giant cells were present, containing similar crystalline material. In some sections, large acicular crystals were present within small bronchioles, and there were multifocal inflammatory aggregates composed of small to moderate numbers of lymphocytes and plasma cells scattered randomly throughout the lung parenchyma.
Morphologic Diagnosis:
Lung: Pulmonary adenomas; Mild to moderate, chronic, multifocal to coalescing, accompanied by histiocytic to granulomatous interstitial pneumonia with intracytoplasmic eosinophilic crystalline material.
Condition:
Pulmonary adenomas and eosinophilic crystaline pneumonia
Contributor Comment:
Eosinophilic crystalline pneumonia is an idiopathic disease that occurs in several different strains of mice, most commonly in those of a C57BL/6 background.(4,8) This disease varies from mild and subclinical to severe, sometimes resulting in dyspnea and death. This disease may occur alone or in conjunction with other pulmonary lesions such as pulmonary adenomas (as in this case), lymphoproliferative disease, allergic lung disease, and parasitic or fungal infections.(1,3) The crystals that are found within the cytoplasm of macrophages and multinucleate giant cells, as well as often free within alveolar spaces and bronchioles, are reminiscent of Charcot-Leyden crystals morphologically, which are products of eosinophil breakdown and eosinophil-related diseases in humans.(3,8) However, crystals in ECP are composed primarily of Ym1 protein, a chitinase-like protein that is secreted by activated macrophages. The exact function of Ym1 protein is poorly understood, but is believed to play a role in host immune defense, eosinophil recruitment, and cell-cell and cell-matrix interactions consistent with tissue repair.(2,3)
JPC Diagnosis:
- Lung: Pulmonary (bronchioalveolar) carcinoma.
- Lung: Pulmonary (bronchioalveolar) adenoma.
- Lung: Bronchioalveolar hyperplasia, multifocal.
- Lung: Pneumonia, interstitial, histiocytic, focally extensive, marked, with intracytoplasmic eosinophilic crystalline inclusions.
Conference Comment:
The slide variation with this case initiated an excellent discussion amongst conference participants regarding the spectrum of lung lesions observed in various sections. The severity of eosinophilic crystalline pneumonia ranges from mild alveolar histiocytosis in some sections to granulomatous pneumonia in others. In some sections, eosinophilic crystals were visible within epithelial cells lining bronchi as well. Additionally, there are also several proliferative bronchoalveolar lesions comprising a continuum from what conference participants considered benign hyperplasia to adenoma to carcinoma. Within nodules of more quiescent hyperplastic/adenomatous cells, there are occasionally foci of cells with a more malignant appearance that were interpreted as representing malignant transformation
Discussion also centered on the current variation in classification of and terminology for lung tumors in the mouse, arising from the lack of a consensus on histologic subtype.(6) In some instances, neoplasms showing lepidic growth along preexisting alveolar structures have been referred to as bronchioalveolar adenoma if < 3mm and bronchioalveolar carcinoma if > 3mm. However, Percy and Barthold, in the 3rd edition of
Pathology of Laboratory Rodents and Rabbits, use the terminology pulmonary rather than bronchioalveolar when describing these neoplasms in mice.5 Interestingly, in human pathology, the term bronchioalveolar carcinoma has recently been dropped from the terminology, and the new (2011) classification scheme uses the terms atypical adenomatous hyperplasia for preinvasive hyperplastic lesions, adenocarcinoma in situ and minimally invasive adenocarcinoma for small (<3 cm) solitary adenocarcinomas with pure or predominant lepidic growth, respectively, and < 5 mm invasion, and invasive adenocarcinoma for tumors with > 5 mm invasion.(7)
References:
1. Guo L, Johnson RS, Schuh CL. Biochemical characterization of endogenously formed eosinophilic crystals in the lungs of mice.
J Biol Chem. 2000;275:8032-8037.
2. Hoenerhoff MJ, Starost MF, Ward JE. Eosinophilic crystalline pneumonia as a major cause of death in 129S4/SvJae mice.
Vet Pathol. 2006;43: 682-688.
3. Marchesi F, Monestiroli SV, Capillo M, et al. Eosinophilic crystals as a distinctive morphologic feature of a hyaline droplet nephropathy in a mouse model of acute myelogenous leukemia.
J Vet Med. 2003;50:103-107.
4. Murray AB, Luz A. Acidophilic macrophage pneumonia in laboratory mice.
Vet Pathol. 1990;27:274-281.
5. Percy DH, Bathrold SW. Rabbit. In:
Pathology of Laboratory Rodents and Rabbits. 3rd ed. Ames, Iowa: Blackwell; 2007:117-118.
6. Rissoto KC, Lucas P, Fan TM. An update on diagnosing and treating primary lung tumors. DVM 360. 1 March 2008.
http://veterinarymedicine.dvm360.com/vetmed/article/ articleDetail.jsp?id=503048&sk=&date=&pageID=2
7. Travis WD, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European RespiratorySociety: International Multidisciplinary Classification of Lung Adenocarcinoma, An Executive Summary.
Proc Am Thorac Soc. 2011;8:381385.
8. Ward JM, Yoon M, Anver MR, et al. Hyalinosis and Ym1/Ym2 gene expression in the stomach and respiratory tract.
Am J Pathol. 2001;158:323-332.