WSC 22-23
Conference 10
Case 1
Signalment:
Unknown gestational age*, intact female, aborted dairy goat fetus (Capra aegagrus hircus)
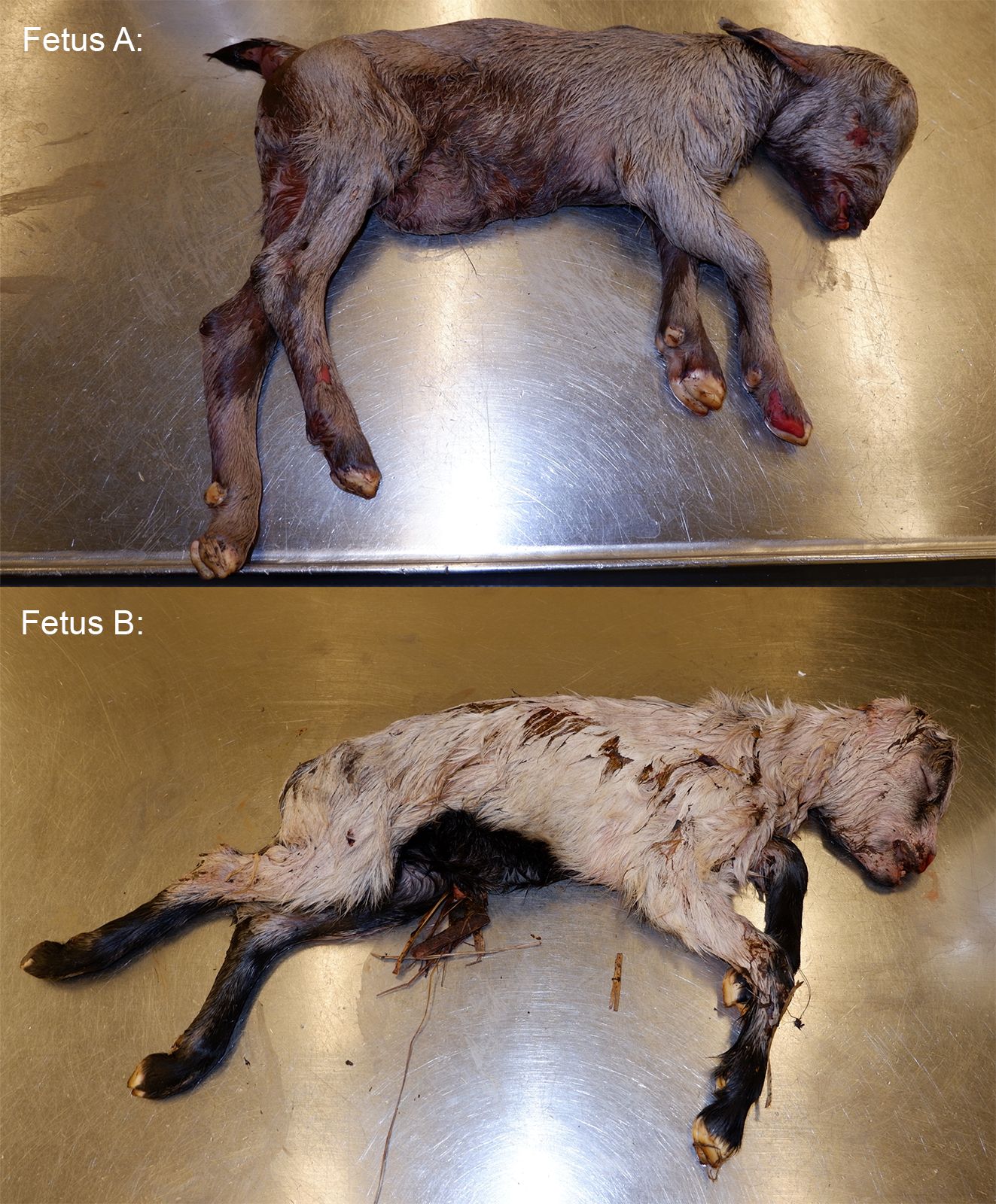
*The animal appears to be nearly full-term on gross examination and measures 46 cm from crown to rump.
History:
None provided with the submission. This animal was submitted with a male twin. No placenta was submitted with the fetuses.
Gross Pathology:
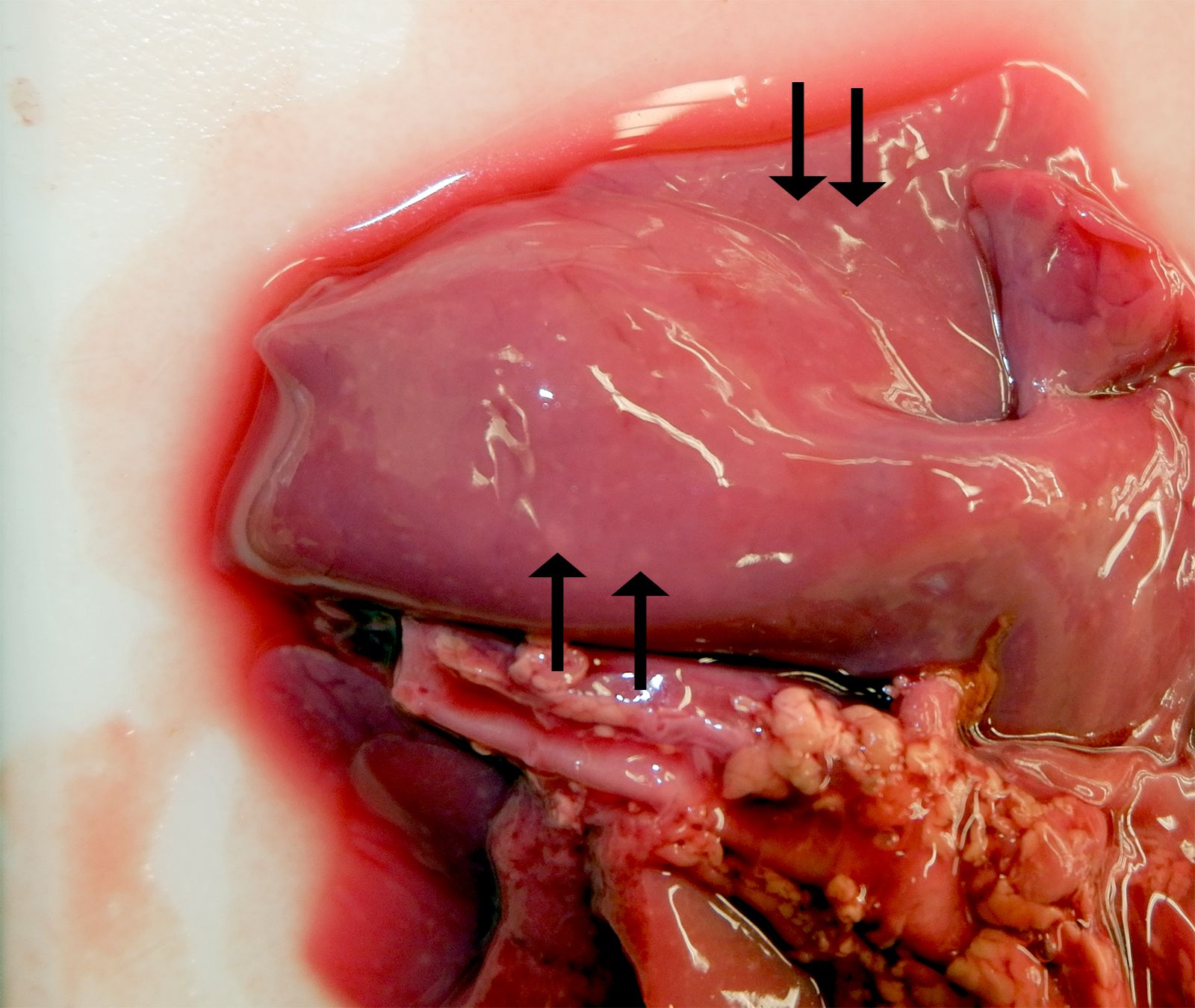
The female twin was grossly unremarkable. The lungs of the male twin had hundreds of disseminated, smooth, white, and flat pinpoint to 2.0 mm nodules within the lung parenchyma and on the pleural surfaces. Lung sections from both twins sank in formalin.
Laboratory Results:
An in-house real time reverse transcriptase multiplex rRT-PCR test using pooled liver had the following result: infectious bovine rhinotracheitis (IBR) was suspect positive, with a Ct of 38; tissues were negative for bovine respiratory syncytial virus (BRSV) and parainfluenza-3 (PI3). Aerobic bacterial culture of lung yielded no growth of pathogens.
Caprine herpesvirus (CapHV-1) PCR testing of a lung sample at Colorado State University Veterinary Diagnostic Laboratory detected nucleic acids (positive).
The viral inclusions did not stain using bovine herpesvirus-1 (infectious bovine rhinotracheitis) antibody (immunohistochemistry).
Microscopic Description:
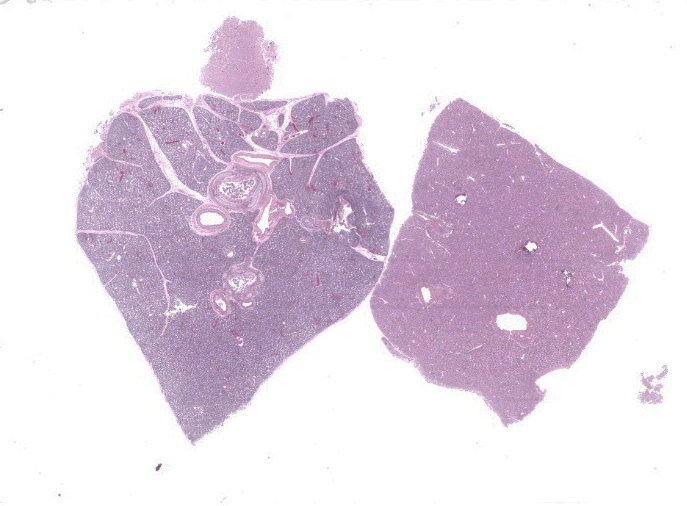
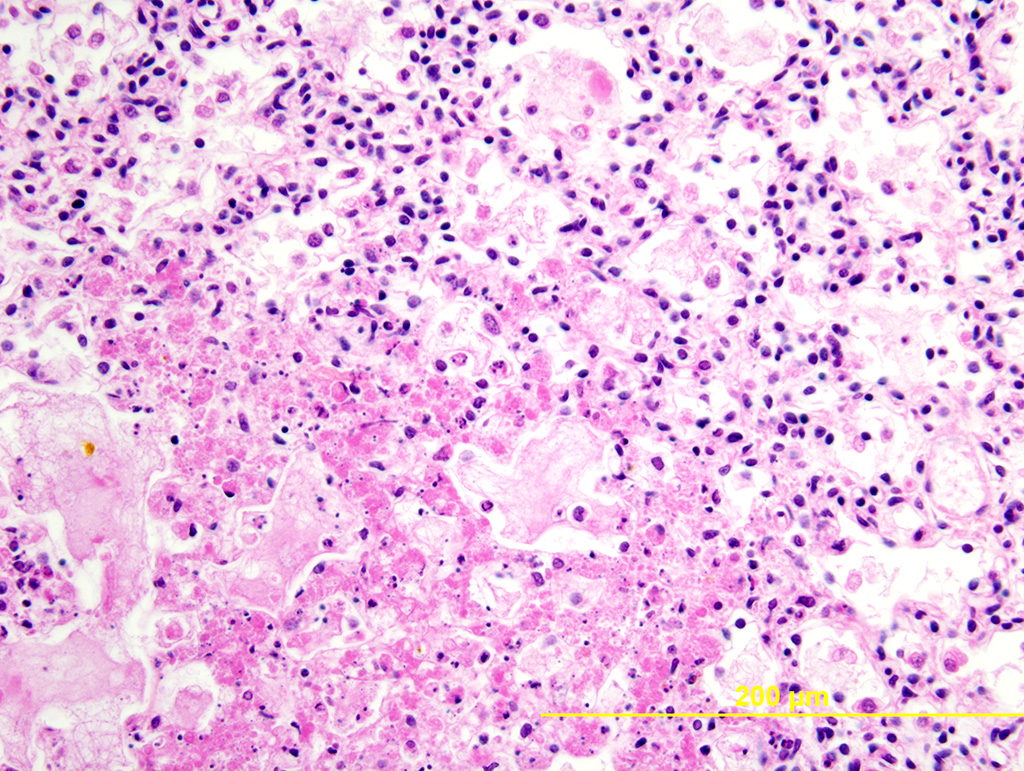
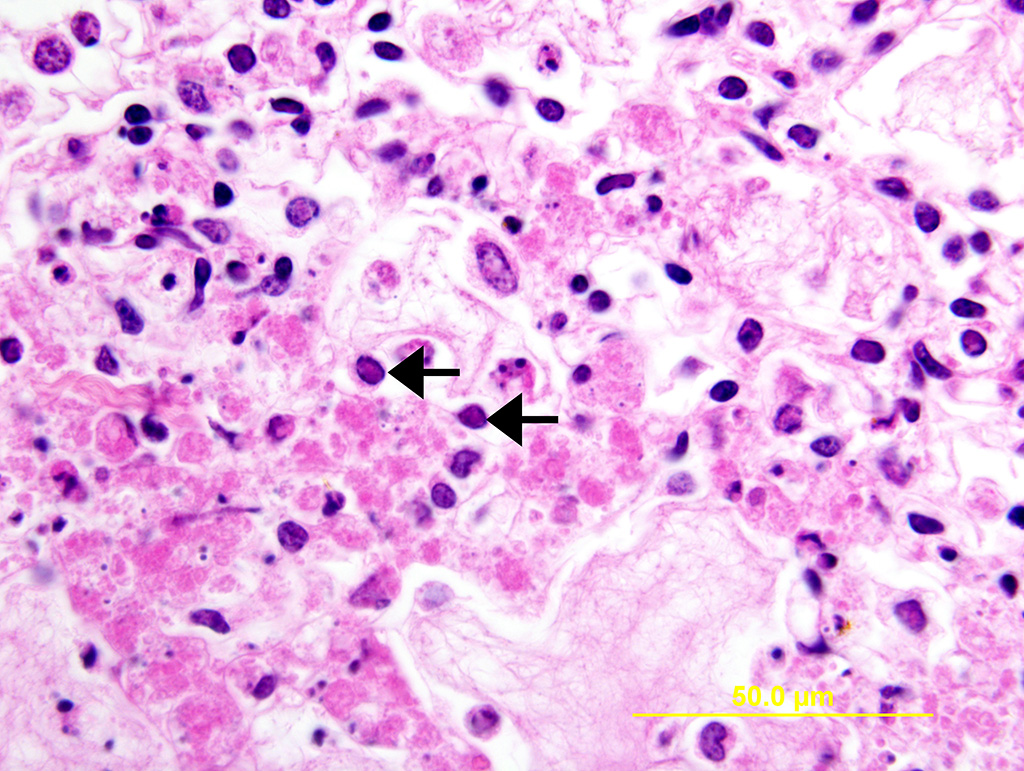
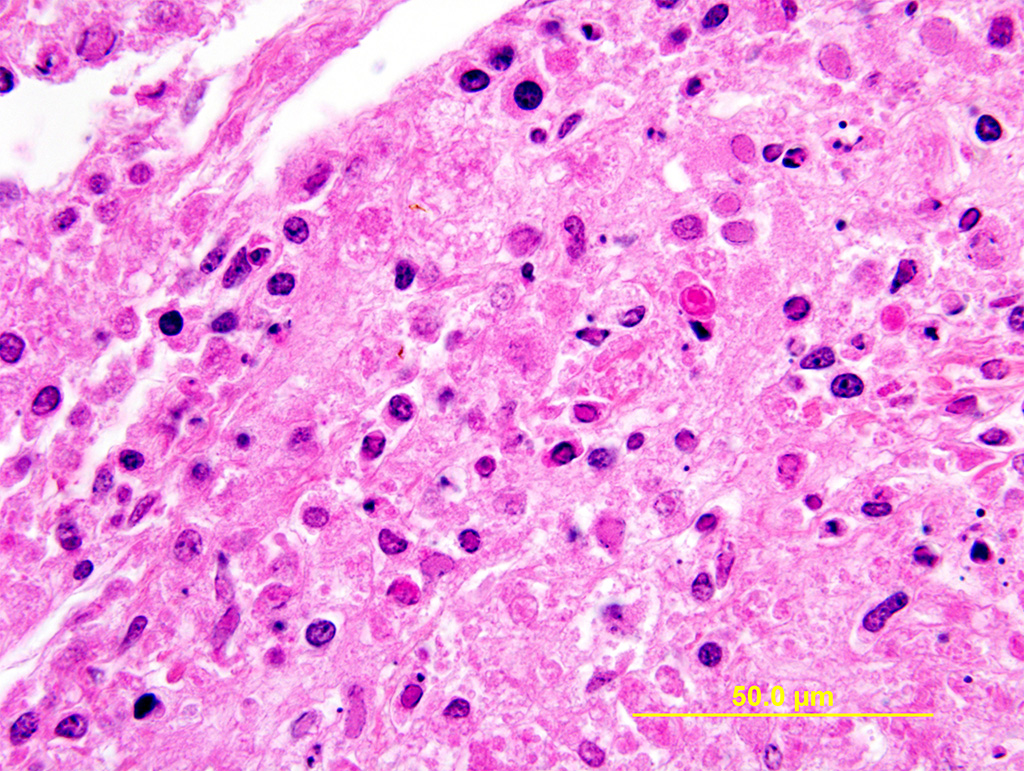
Lung: Multiple random foci of necrosis that measure up to 1 mm wide disrupt the pulmonary parenchyma. The alveolar septa are markedly expanded by flocculent pink cell debris, karyorrhectic material and fibrin (coagulative necrosis). Few macrophages, neutrophils, and erythrocytes are admixed. Alveoli are filled with fine pink fibrillar material (fibrin) and pale pink fluid. At the periphery of these foci are degenerating cells with usually a single, eosinophilic intranuclear inclusion that measures approximately 5 um and peripheralizes the chromatin.
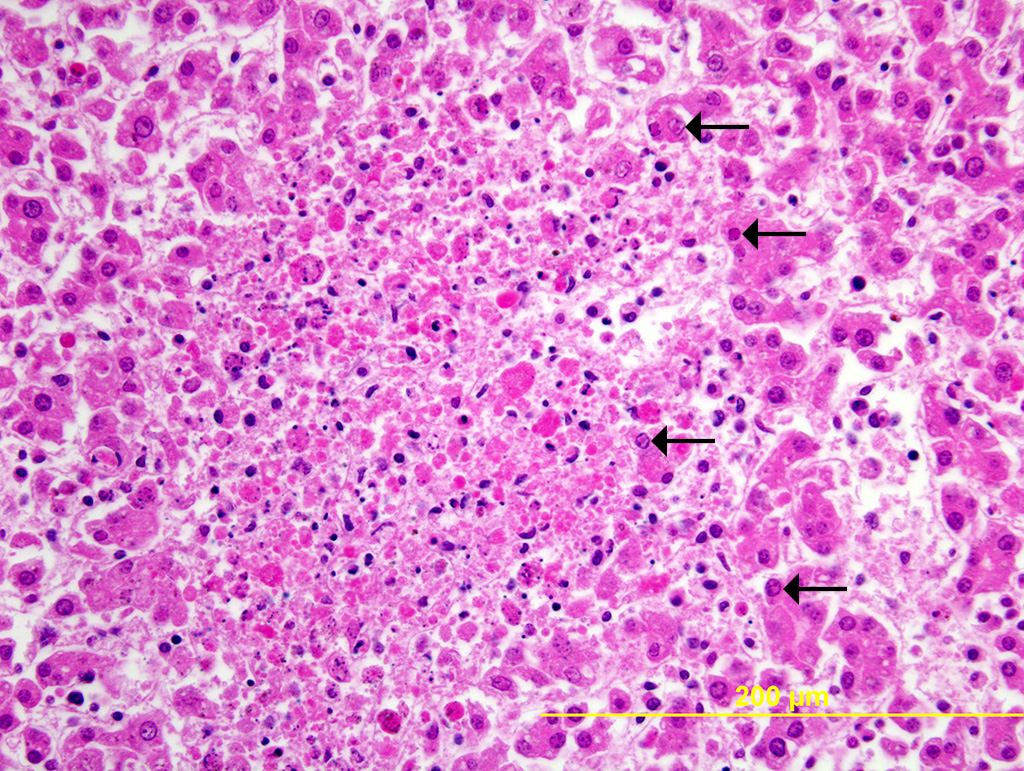
Liver: Multiple random foci of hepatic necrosis disrupt the parenchyma and are composed of globular hypereosinophilic cell fragments and karyorrhectic debris admixed with small numbers of macrophages. Hepatocytes along the periphery often have eosinophilic intranuclear inclusions that peripheralize chromatin. Macrophages in and around these nodules have small amounts of globular, dark brown, intracytoplasmic pigment (presumably hemosiderin)
Contributor’s Morphologic Diagnoses:
- Lung: multifocal, random, moderate, necrotizing pneumonia with intranuclear viral inclusion bodies
- Liver: multifocal, random, mild, necrotizing hepatitis with intranuclear viral inclusion bodies
Contributor’s Comment:
Caprine herpesvirus 1 (CpHV-1) is globally distributed alphaherpesvirus that occasionally causes disease and is less-commonly implicated as a cause of infectious abortion.2,6,7,10,11 Clinical manifestation of disease depends on the age of the infected animal. In 1- to 2-week-old kids, a generalized disease with severe gastrointestinal lesions predominates.1,3,4,6 In contrast, infected adults are often clinically silent or have genital tract infections characterized by vulvovaginitis or balanoposthitis; more rarely do respiratory tract infections and abortions occur. 2,6,7,10,11 Typically, does that abort are subclinically infected; the only manifestation of infection can be late term abortions, stillbirths, or even abortion storms at a herd level. 2,7,10,11 Transmission is thought to be from direct contact during coitus.10 The source of infection can be challenging to determine, as alphaherpesviruses can remain latent in the trigeminal ganglia or other tissues.2
The observed herpetic inclusions are consistent with typical Cowdry type A inclusions, which are characterized by large, intranuclear, and eosinophilic to amphophilic inclusions that marginalize the chromatin.2,3,7 With CpHV-1, inclusions are often at the periphery of necrotic foci.7 The adrenal glands, liver, lungs, and kidneys are the organs that most commonly contain inclusions.7 With advanced autolysis, inclusions can be challenging to identify; however, examination of the adrenal gland appears to be a reliable site of inclusion identification.12 In this case, inclusions were found in the lung, liver, thymus, and adrenal glands but were most numerous within the lung and liver, as submitted.
The other distinguishing histopathologic feature for this infectious abortifactive agent is widespread necrotic foci that are most common within the lungs, adrenal gland, and thymus.2,7,12 Other affected organs can include the kidney, intestine, lymph nodes, and spleen.7,12 Thymic necrosis is associated with clusters of macrophages containing the typical Cowdry type A inclusion bodies, which, interestingly, is similar to what is observed in infected neonatal kids.3,11
The pathogenesis has yet to be completely elucidated, but it is thought to be associated with viremia of the dam after respiratory or genital colonization.2,11,12 This results in leukocyte trafficking or hematogenous spread to the uterus where placental endothelium, mesenchyme, and trophoblasts become infected.12 Death is due to tissue destruction within the fetus and placenta.12 Viremic potential varies with individual strain, which may further contribute to the rarity of these abortions.11,12
Other, more common, causes of caprine abortion should be considered first when presented with a late term caprine abortion, including: Chlamydophila abortus, Coxiella burnetii, Toxoplasma gondii, and Listeria monocytogenes.2,7 In this case, however, these agents were not identified via histologic examination or ancillary testing.
The “suspect” result of the rtPCR for infectious bovine rhinotracheitis suggests that this causative virus has genetic homology with bovine herpes virus-1 (BHV-1). It has been previously shown that CpHV-1 and BHV-1 are closely related and often antibodies will often cross-react on fluorescent antibody or immunohistochemical assays.2,6,7 Furthermore, goats can seroconvert against bovine herpesvirus 1, although no BHV-1 induced abortions have been identified in goats, to the author’s knowledge.12 The fetal lesions of CpHV-1 and BHV-1 are essentially identical.12 Bovine herpesvirus-1 abortions tend to consistently have a necrotizing vasculitis within small vessels of the placental villi.12 Unfortunately, the placenta was not submitted with this case for evaluation.
The male twin had histopathologically similar lesions in the lungs, adrenal glands, and thymus.
Contributing Institution:
University of Illinois at Urbana-Champaign, Veterinary Diagnostic Laboratory
JPC Diagnosis:
1. Lung: Pneumonia, necrotizing, multifocal, mild to moderate, with intranuclear viral inclusions.
2. Liver: Hepatitis, necrotizing, multifocal, mild to moderate, with intranuclear viral inclusions.
JPC Comment:
This week’s moderator, LTC Joseph Anderson, described a few of the histologic features which can indicate that lung samples originated from fetal or neonatal animals. In neonatal rats, the terminal bronchiolar epithelial cells are vacuolated due to intracytoplasmic glycogen accumulations, a feature which was also present in this case.5 Additionally, developing lung in the saccular stage has thickened airway walls with a large amount of stroma.5 In this case, there are also squamous epithelial cells within the alveolar lumina which were introduced by amniotic fluid.
Caprine herpesvirus-1 is a double-stranded DNA virus that was first isolated in California and Switzerland in the 1970s and has subsequently achieved a world-wide distribution.9 Serologic testing for CpHV-1 involves measuring BoHV gB and gE ELISA reactivity; positive gB and negative gE results indicate CpHV-1 infection.1,13 Serologic studies have elucidated risk factors for CpHV-1 infection, which include large herd size, meat or mixed production breeds, older age, and caprine arthritis-encephalitis viral co-infection.1 A separate study in France confirmed that larger herds were more likely to be seropositive, and in these herds, up to 51% of animals were seropositive.13 The correlation of older age with seropositivity suggestive of prolonged exposure and viral re-circulation within the herd.1,13
CpHV-1 has been the subject of recent research on therapeutic oncolytic viruses, which can replicate in and destroy neoplastic cells without harming normal cells. A few viruses, such as adenoviruses, herpesviruses, and reoviruses, have been investigated for their oncolytic properties.4 Oncolytic herpes simplex virus 1 (oHSV-1) is the first approved oncolytic virus and targets advanced stage, non-resectable melanomas in humans.4 As it is based on a naturally-occurring human virus, oSHV-1 can cause disease in non-neoplastic tissue as well; thus there is interest in using wild-type viruses that do not normally infect humans. Additionally, humans do not have pre-existing immunity to this virus and thus may be more susceptible to therapeutic infection.4 Several alpha herpesviruses, including BoHV-1, equine herpesvirus 1, and now CpHV-1 have demonstrated oncolytic properties.8 CpHV-1 can replicate in and increase apoptosis in several human cancer cell lines.8 CpHV-1 has promising effects in the treatment of mesothelioma, which has an average survival time of less than 12 months in humans.4 A recent study showed that the virus induced apoptosis in neoplastic cell cultures, halted cell cycle progression, and had synergistic effects with cisplatin, the current chemotherapeutic of choice for mesothelioma, with minimal effects on normal mesothelial cells.4
References:
- Bortelini S, Rosamilia A, Caruso C. A cross-sectional study to identify a set of risk factors for caprine herpesvirus 1 infection. BMC Veterinary Research. 2108; 14(94):1-7.
- Chénier S, Montpetit C, Hélie P. Caprine herpesvirus-1 abortion storm in a goat herd in Quebec. Can Vet J. 2004;45:241-243.
- Cowdry, EV. The problem of intranuclear inclusions in viral diseases. Arch Pathol. 1934;18(4):527-542.
- Forte IM, Indovina P, Montagnaro S. The Oncolytic Caprine Herpesvirus 1 (CpHV-1) Induces Apoptosis and Synergizes with Cisplatin in Mesothelioma Cell Lines: A New Potential Virotherapy Approach. Viruses. 2021; 13:2458-2472.
- Greeley MA. Respiratory System. In: Parker GA, Picut CA, eds. Atlas of Histology of the Juvenile Rat. Cambridge, MA: Elsevier; 2016. 90-91.
- Hao F, Mao L, Li W, et al. Epidemiological investigation and genomic characterization of Caprine herpesvirus 1 from goats in China. Infect Genet Evol. 2020;79:104168.
- Moeller RB. Chapter 3: Disorders of Sheep and Goats. In: Njaa BL, ed. Kirkbride’s Diagnosis of Abortion and Neonatal loss in Animals. 4th West Sussex, UK: Wiley-Blackwell. 2012; 51-52, 55-57.
- Montagnaro S, Damiano S, Ciarcia R. Caprine herpesvirus 1 (CpHV-1) as a potential candidate for oncolytic virotherapy. Cancer Biology and Therapy. 2019; 20(1): 42-51.
- Osterreider K. Herpesvirales. In: MacLachlan NJ, Dubovi EJ, eds. Fenner’s Veterinary Virology. 5th ed. Cambridge, MA: Elsevier. 2017; 189-191, 202.
- Pollock JM, Schofield MJ, Porter C, Miner K, Blash S, Gavin WG. Caprine herpesvirus 1: A successful eradication program in dairy goat herd. Small Ruminant Res. 2019;170:8-11.
- Roperto F, Pratelli A, Guarino G, et al. Natural Caprine Herpesvirus 1 (CpHV-1) Infection in Kids. J Comp Path. 2000;122:298-302.
- Schlafer DH, Foster RA. Chapter 4: Female Genital System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, Volume 3. 6th St. Louis, MO, USA. Elsevier; 2016:433-435.
- Suavet F, Champion JL, Bartolini L. First Description of Infection of Caprine Herpesvirus 1 (CpHV-1) in Goats in Mainland France. 2016; 5(17):1-13.