Signalment:
Adult male vervet monkey,
Chlorocebus aethiopsAfter arriving into the CDC quarantine, this animal was noted to have labored breathing and coughing and died on the morning of the next day. There were no experimental procedures performed on this animal.
Gross Description:
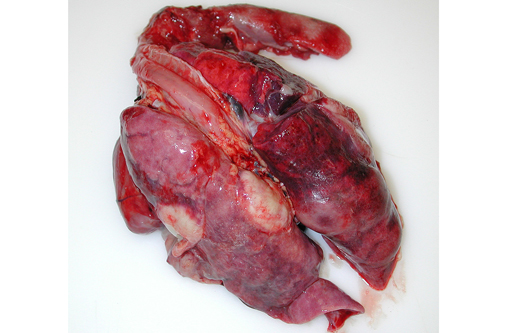
A necropsy was performed as per the CDC quarantine animal necropsy protocol. While opening the diaphragm to enter the thoracic cavity, abundant yellowish-tan, opaque viscous liquid (pus) poured out. The lungs were mottled dark brown to tan to dull red, markedly consolidated, had a consistency similar to that of liver (hepatization of lung), and did not collapse with loss of thoracic cavity negative pressure. Multifocal, tan to white, well demarcated, variably sized pus-filled abscesses reaching up to 2.5 cm in diameter were found on cut sections. Only about 10 percent of apparently normal, remaining pulmonary parenchyma was present. The pleura was thickened and multifocally adhered to the dorsal and lateral thoracic walls. A variably thick layer of pus admixed with dull red to brown fibrillar material (fibrin) was present on the pleural surface and in the thoracic cavity. Mucus admixed with small plugs of pus were present in the distal bronchial lumina. The tracheobronchial lymph nodes were prominent.
Gross Morphologic Diagnoses: Pleuropneumonia, diffuse, chronic, severe, suppurative with abscessation and thoracic adhesions
Histopathologic Description:
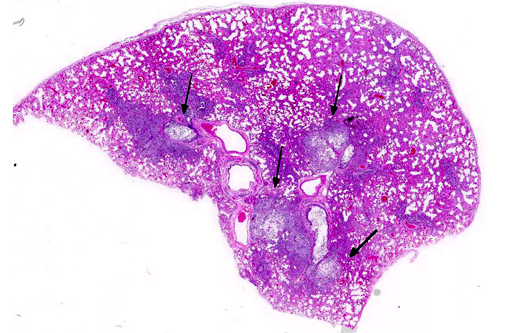
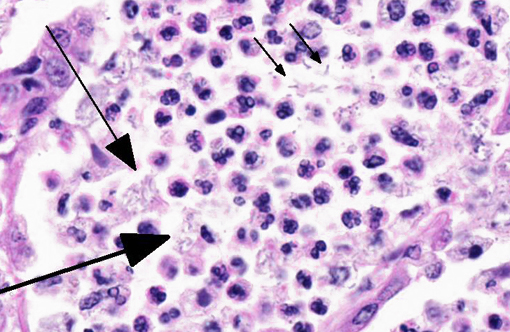
The pulmonary architecture was extensively obscured by abundant suppurative inflammatory cellular infiltrate admixed with edema, necrotic debris and fibrin. Bronchioles and alveolar spaces were filled by numerous neutrophils, many degenerate, which often transmurally infiltrated and effaced alveolar and bronchiolar walls. The affected bronchioles were lined by attenuated epithelial cells lacking cilia and multifocally the epithelial lining was denuded. Most of the blood vessels were surrounded by thick fibrin strands and clear spaces (edema). The alveolar capillaries are congested and small hemorrhages are scattered throughout the pulmonary parenchyma. The pleura was expanded up to 150 microns in thickness by fibrin. Frequently within the airspaces, 1x2-4 micron bacterial rods occasionally arranged in chains were seen extracellularly or within macrophage cytoplasm.
Gram stain: Numerous gram negative 1x2-4 micron single or chains of bacilli were present at the areas of inflammation.
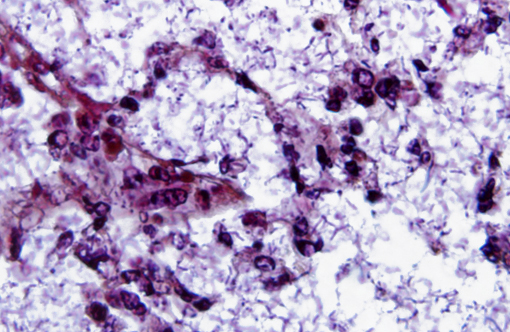
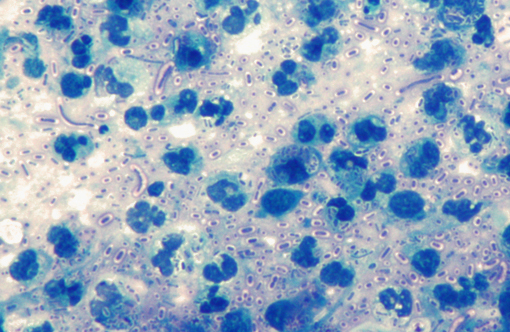
Acid fast stain: The bacterial rods were acid fast negative, but acid fast stain showed a prominent blue stained bacterial capsule
Morphologic Diagnosis:
Pneumonia, bronchointerstitial, diffuse, severe, subacute, fibrinosuppurative with intralesional gram negative bacteria
Lab Results:
Bacterial culture from the lung -
Klebsiella pneumonia 3+ Filoviral ELISA negative
Condition:
Klebsiella pneumonia, vervet monkey
Contributor Comment:
Klebsiella pneumonia is a gram-negative, aerobic, encapsulated, nonmotile rod-shaped bacteria of the family
Enterobacteriaceae, which are ubiquitous in nature. It is one of the most important nosocomial bacterial infections in humans, which accounts for a significant proportion of urinary tract infections, pneumonia, septicemias, and soft tissue infections.(4) As opportunistic pathogens,
Klebsiella spp. primarily attack immunocompromised individuals. It is also a frequent cause for pneumonia in nonhuman primates. Differentials for bacterial pneumonia in nonhuman primates include
Mycobacterium tuberculosis, Burkholderia sp.,
Klebsiella sp.,
Staphylococcus sp.,
Corynebacterium sp.,
Streptococcus sp.,
Nocardia sp.,
Actinomyces sp, among others.
Based on the pathological findings, it is presumed that this animal was infected with
K. pneumoniae and developed pneumonia prior to shipping to Wake Forest, although the transportation-associated stress likely aggravated the condition. Stress associated with shipping and transportation is known to increase the incidence of this infection. Multisystemic abscess formation in African green monkeys caused by invasive, hypermucoviscosity phenotype
Klebsiella pneumonia has been reported.(6) Serotyping based on the capsular antigen type is the technique for further characterizing
Klebsiella spp.
K. pneumoniae characteristically produce vast amounts of capsular polysaccharide covering the bacterial surface.(5) K1 and K2 serotypes are most virulent and they were found to be significantly more resistant to phagocytosis than non-K1/K2 isolates.(1,5) The thick polysaccharide capsule (K antigen) of these bacteria creates a barrier that prevents opsonization and phagocytosis. In this case, capsular serotyping was not performed. This animal also had meningitis, epicarditis and renal arterial thrombosis which suggest bacterial septicemia. Also moderate myeloid hyperplasia in the bone marrow was observed as expected in bacterial infections.
JPC Diagnosis:
Lung: Bronchopneumonia, fibrinosuppurative, chronic, multifocal, severe, with numerous intra- and extracellular bacilli.
Conference Comment:
This is a classic case of the primate form of shipping fever, a disease affecting both New and Old World primates and can lead to sepsis and rapid death, which likely occurred in this case. The organisms are numerous in this example, and the thick capsule so important to its pathogenesis is beautifully depicted in the image of the acid fast stain submitted by the contributor. This capsule also likely plays a role in the increased invasiveness and pathogenicity of the hypermucoviscosity phenotype (HMV), which carries greater resistance to oxidative-mediated killing and imparts a higher level of cytotoxicity upon the host.(2) HMV was first isolated from fatal human infections and was only recently described in nonhuman primates; it characteristically causes rampant abscesses affecting multiple organs.(6)
K. pneumonia is associated with disease in other lab animal species as well, including enterotyphilitis and bronchopneumonia in rabbits, polyserositis and bronchopneumonia in guinea pigs, and lymph node and renal abscesses in mice and rats.(3) The closely related
K. oxytoca causes suppurative endometritis and dermatitis readily in mice.(3) Among domestic species,
K. pneumoniae causes urinary tract infections (dogs), mastitis (ruminants), cerebral abscesses (ruminants) and pneumonia (dogs).(7)
References:
1. Chuang YP, Fang CT, Lai SY, Chang SC, Wang JT. Genetic determinants of capsular serotype K1 of Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis 193: 645-654, 2006
2. Cox BL, Schiffer H, Dagget G Jr, et. al. Resistance of Klebsiella pneumonia to the innate immune system of African green monkeys. Vet Microbiol. 2015;176(1-2):134-142.
3. Percy DH, Barthold SW. Pathology of Laboratory Rodents. 3rd ed. Ames, IA: Blackwell Publishing; 2007:64,152,222,275.
4. Podschun R, Ullmann U: Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11: 589-603, 1998
5. Struve C, Bojer M, Nielsen EM, Hansen DS, Krogfelt KA. Investigation of the putative virulence gene magA in a worldwide collection of 495 Klebsiella isolates: magA is restricted to the gene cluster of Klebsiella pneumoniae capsule serotype K1. J Med Microbiol 54: 1111-1113, 2005
6. Twenhafel NA, Whitehouse CA, Stevens EL, Hottel HE, Foster CD, Gamble S, Abbott S, Janda JM, Kreiselmeier N, Steele KE: Multisystemic abscesses in African green monkeys (Chlorocebus aethiops) with invasive Klebsiella pneumoniae--identification of the hypermucoviscosity phenotype. Vet Pathol 45: 226-231, 2008
7. Zachary JF, McGavin MD. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier Mosby; 2012:524,637,801,1124.