Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 12
12 December, 2018
CASE IV 16H94455 or blank (JPC 4100730)
Signalment: Canine, Golden Retriever, 0.5 years old, male
History: This dog was purchased through a breeder at 7 weeks of age. At around 4 months of age, the dog started having uncontrolled watery−mucoid diarrhea that was large volume and frequent. He was placed on Metronidazole and Proviable and needed to stay on the medication as diarrhea would return when the drugs were not used. The dog was diagnosed with carpal flexure deformity at 3 months of age. Radiographs of the cervical spine and hips revealed mild hip dysplasia and shortened distal cervical vertebral bodies. The dog developed urinary accidents (normal volume) at home. On presentation to Iowa State University Teaching Hospital, the dog had lateral strabismus, vertical nystagmus, ataxia that was worse in hindlimbs with normal reflexes and placement and hopping, head tremor, and rectal mucosa felt very irregular on rectal palpation. The owner did not allow many additional tests; only creatine kinase, which was mildly increased at 378, and blood smear which showed possible granules in lymphocytes. The dog’s condition deteriorated over a week and was euthanized.
Gross Pathology: Small intestine: A small number (approximately 10) of 2−5 cm in length and 2 mm in diameter, white, elongate nematodes with tapered ends (roundworms) were present multifocally within the small intestinal lumen.
Laboratory results: Hepatic b-hexosaminidase A (HexA) enzyme activity was assayed. Enzyme activity levels were increased compared to the normal canine liver standard.
Electron microscopy was performed on sections of brain. The vacuoles appeared as round to ovoid membrane bound bodies with, most frequently, internal concentric whorls of membrane with a periodicity of 6-8 nanometers.
Microscopic Description:
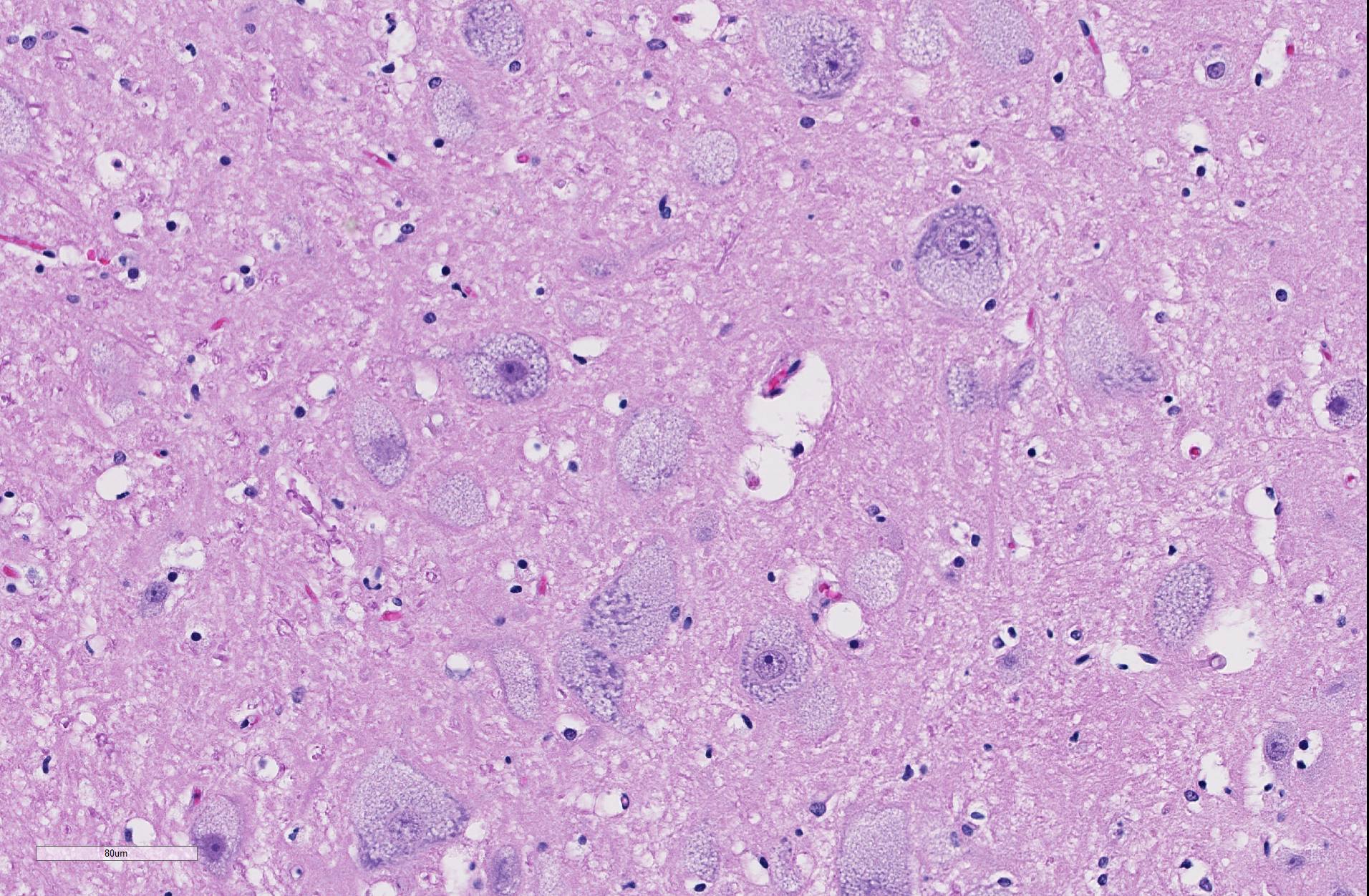
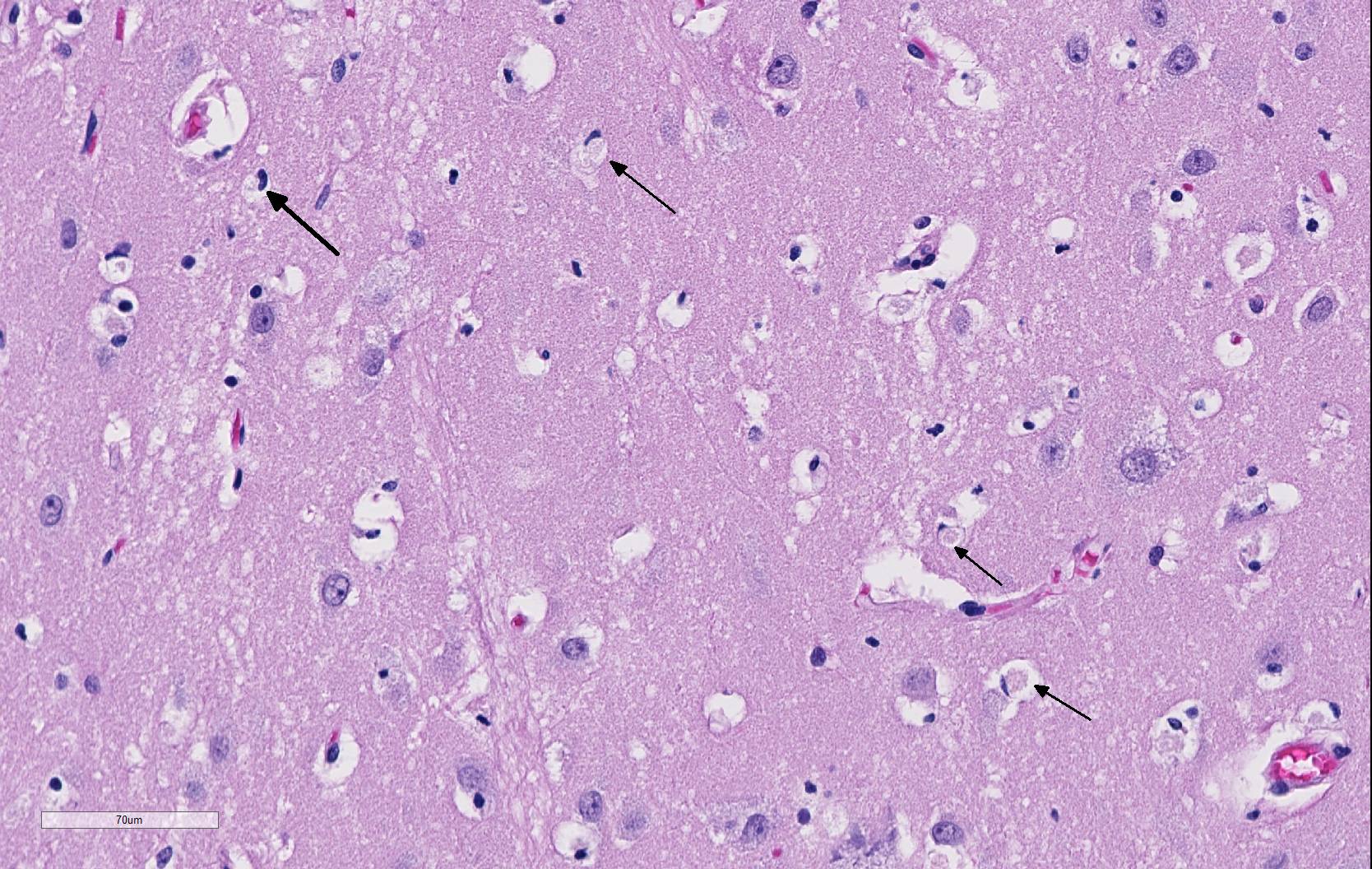
Spinal cord (cervical; thoracic;lumbar, sacral): All spinal cord segments are similarly affected. Diffusely, neurons are variably expanded by abundant finely vacuolated cytoplasm, which often peripheralizes the nucleus and Nissl substance. Multifocal neurons contain larger vacuoles, up to 8 microns in diameter, containing pale eosinophilic material. Some neurons contain both foamy microvacuolated cytoplasm and larger vacuoles. Glia appear unaffected. White matter and nerve rootlets contain scattered dilated and often empty axon sheaths. Meninges, blood vessels − no specific pathologic findings.
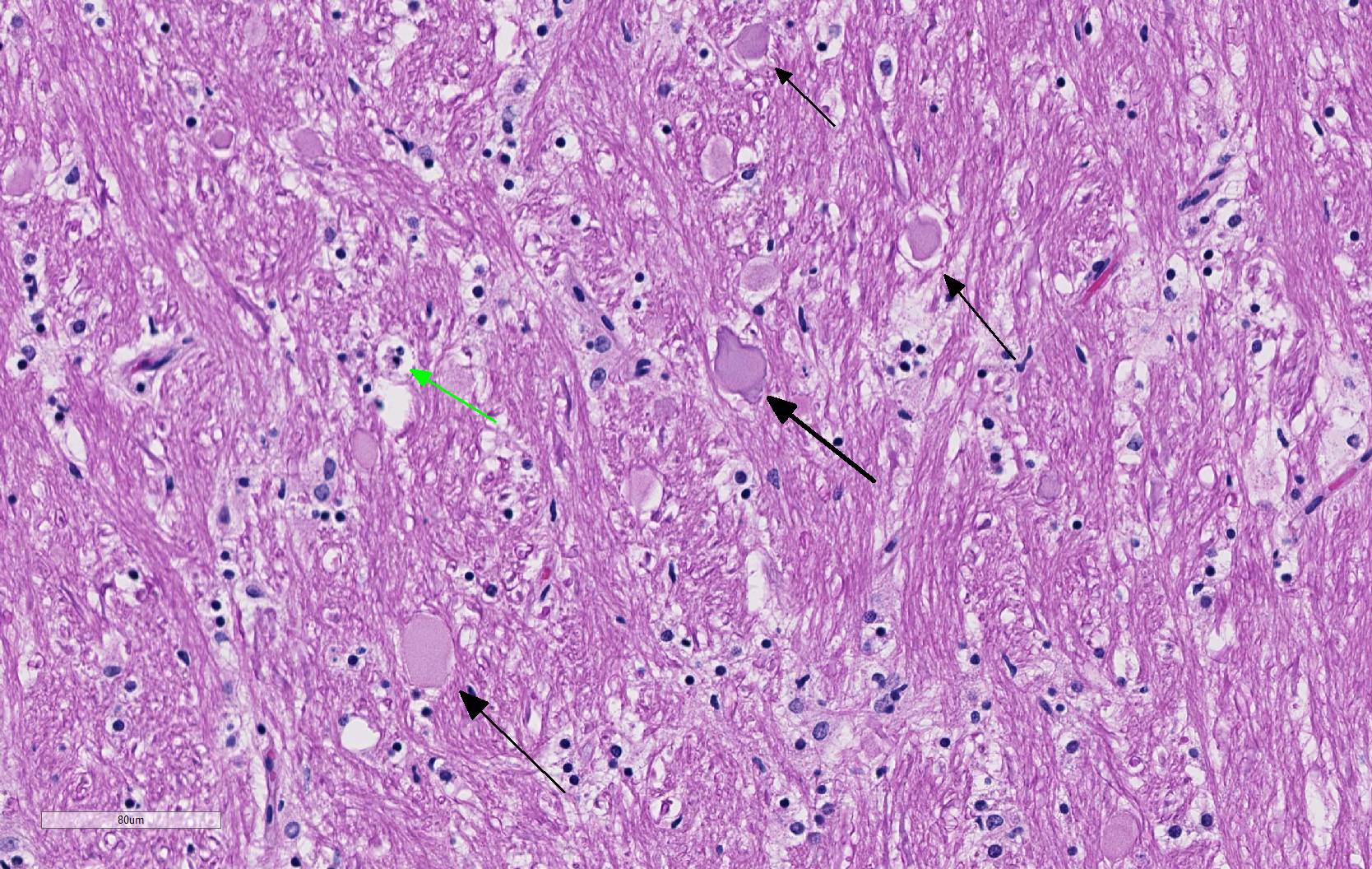
Brain: thalamus, hippocampus; level of caudate nucleus; cerebrum; midbrain; cerebellum; rostral cerebral cortex; pons: diffusely, neurons are moderately to markedly expanded by similar microvacuolated cytoplasm, which often peripheralizes the nucleus and occasionally extends into and expands proximal processes. Infrequently, neurons also contain larger vacuoles up to 8 microns in diameter. Microglia are subjectively increased in number. There is rare neuronal necrosis, and rare (two) blood vessels are cuffed by few lymphocytes. White matter is mildly vacuolated with occasional spheroids and few vacuolated cells (small neuron or astrocyte morphology). Neuronal vacuolation in diencephalon and brainstem nuclei is especially pronounced. The caudate nucleus is regionally strikingly hypercellular with vascular proliferation and gliosis − extant neurons are similarly vacuolated. Purkinje cells are similarly vacuolated; PC density appears normal. Cerebellar nuclei are hypercellular with marked gliosis, similarly vacuolated neurons, and moderate numbers of spheroids in white matter tracts. Meninges, blood vessels − no specific pathologic findings.
Eye: Retinal ganglion cells are similarly enlarged and vacuolated with margination of the nucleus.
Intestine (duodenum, colon): Myenteric and submucosal plexus neurons occasionally appear enlarged with microvacuolated clear to eosinophilic cytoplasm with marginated nuclei.
Urinary bladder: Ganglion cells within the bladder wall are similarly enlarged and vacuolated.
Liver: Diffusely, hepatocytes contain coarse, coalescing vacuoles with ragged margins.
Mesenteric ganglion: Ganglion cells are markedly distended by similar microvaculated cytoplasm as described above.
No significant microscopic changes were identified in the following tissues: kidney, colon, skeletal muscle (rare protozoal cysts), ileum, cecum (advanced mucosal autolysis), trachea, sciatic nerve, prostate, testis, tongue, heart, lung (marked congestion with RBC extravasation), spleen (moderate to advanced autolysis), thymus, thyroid gland, adrenal gland, pituitary (pars distalis), mandibular salivary gland.
Contributor’s Morphologic Diagnoses:
Brain, spinal cord, mesenteric ganglia: Neuronal cytoplasmic vacuolation, diffuse, moderate to severe.
Retina: Retinal ganglion cell vacuolation, cytoplasmic, diffuse, moderate.
Small intestine: Mild endoparasitism (roundworms)
Contributor’s Comment: Lysosomal storage diseases (LSD) are inherited (often autosomal recessive) or acquired (from ingested plant or other toxins) disorders that result in accumulation of macromolecules in lysosomes due to defective catabolism.1,2 Most are due to deficient or inactive hydrolytic enzymes or defects such as inactive proteins, impaired posttranslational processing, lack of enzyme activator, lack of substrate and lack of transport proteins.3 Types of LSDs of veterinary importance include: Sphingomyelin lipidosis (e.g., GM1 and GM2 gangliosidosis and globoid cell leukodystrophy), glycoproteinoses (alpha and beta mannosidosis, l-fucosidosis), mucopolysaccharidoses (MPSI, MPSII, MPS III, VI, and VII), glycogenoses (alpha 1, 4 glucosidase), and neuronal ceroid lipofuscinoses (infantile, late infantile, juvenile, and adult variants).
Microscopic changes in this dog were consistent with a lysosomal storage disease. Vacuolation was predominantly, if not solely, intraneuronal affecting the central and autonomic nervous systems, and retina. Hepatocellular vacuolation was also present, but vacuole morphology appeared more consistent with glycogen than a storage disease enzyme product. Biochemical and/or genetic testing is required for diagnosis of the specific type of LSD.
By electron microscopy, vacuoles appeared most commonly as concentric lamellated inclusions, most typical of the gangliosidoses. Restriction of disease to the nervous system in this case makes a GM2-gangliosidosis more likely. GM2 gangliosidoses reported in animals include Tay-Sachs/variant B (caused by HEXA mutations), Sandhoff/variant 0 (HEXB mutations), and GM2 activator deficiency/Variant AB (GM2A mutations). The finding of increased HexA activity in this case is inconsistent with Tay-Sachs and Sandhoff. In the AB variant, hexosaminidase activity is retained, but there is deficiency of an activator protein (GM2A) that is required for degradation of GM2 ganglioside by HexA. The AB variant has been reported in a Japanese Spaniel.5 Additionally, a novel HEXA missense mutation has been recently identified in Japanese Chin dogs that have a phenotype similar to the AB variant.6 DNA was isolated from this dog and is being tested for known LSD mutations.
Contributing Institution:
https://vetmed.iastate.edu/vpath
JPC Diagnosis: Cerebrum: Neuronal and glial vacuolation, diffuse, marked, with gliosis.
JPC Comment: GM2 ganglioside is a cell membrane glycolipid which is catabolized by the action of N-acetylyhexosaminidase and GM2 activator (GM2A) protein. N-acetylyhexosaminidase exists as a dimer in two forms, hexosaminidase A and hexosaminidase B. Improper functioning of either one of these subunits, or GM2A, results in accumulation of GM2 gangliosides in the lysosomes of neurons leading to a progressive deterioration of the central nervous system (GM2 gangliosidosis, monosialoganglioside gangliosidosis). Tay-Sachs disease of humans is a GM2 gangliosidosis caused by a mutation in the HexA gene and deficienct production of the hexosaminidase A dimer, while Sandhoff disease is caused by mutation of the Hex B gene and deficiency of both hexosaminidase A and B dimers.4 GM2 gangliosidosis has been previously described in toy poodles, German shorthaired pointers and Japanese Spaniel dogs, domestic short haired and Korat cats, Jacob sheep, Yorkshire pigs, and Muntjak deer.
Three forms of GM2 gangliosidosis are defined based on the age of onset and subsequent disease severity a) infantile (classical), b) juvenile, and c) adult onset (a more heterogeneous progression. The most frequent initial signs are developmental arrest, abnormal startling responses, and low muscle tone, ultimately progressing to seizures, blindness, and coma. Gross lesions in affected animals may include macrocephaly, cortical atrophy, broadening of gyri and shallow sulci. 4 Histologic lesions include neuronal vacuolation with displacement of Nissl substance. As gangliosides figure prominently in synaptic transmission, spheroids may be numerous in white matter, as seen in this case. Cytoplasmic vacuolation may be seen in other cells throughout the body including hepatocytes, leukocytes, renal tubular epithelium, pancreatic acinar cells, and corneal stromal cells (corneal clouding has been identified grossly in Portuguese water dogs and cats).
Recent therapeutic studies in treatment of feline GM2-gangliosidosis cats resembling Sandhoff’s disease have shown promise in ameliorating the signs of disease and extending life span. Treatment requires intrathalamic or intraventricular injection of an adenovirus associated virus vehicle encoding both the human HEX A and HEX B genes. Sandhoff-type cats were treated between 4 and 6 weeks of age prior to the onset of clinical signs. Treated cats in this study demonstrated increase Hex activity up to 4-fold, had twice the life-span of untreated cats, and demonstrated up to 90% decreased GM2 ganglioside storage in all CNS regions analyzed.1
The attendees noted that in the submitted sections of slides, it appears that microglia cells are also swollen by vacuolated cytoplasm, which is not inconsistent with a clinical diagnosis of GM2-gangliosidosis, in which vacuolation of perivascular macrophages and glial cells has been described.
References:
- Bradbury AM, Gurda BL, Casal ML, Ponder KP, Vite CH, Haskins ME: A review of gene therapy in canine and feline models of lysosomal storage disorders. H Gene Ther Clin Devel 2015; 26:27-37.
- Cummings JF, Wood PA, Walkley SU, de Lahunta A, DeForest ME: GM2 gangliosidosis in a Japanese Spaniel. Acta Neuropathol, 1985; 67(3):247-253.
- Haskins ME, Giger U, Patterson DF. Animal models of lysosomal storage diseases: their development and clinical relevance. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 years of FOS. Oxford: Oxford PharmaGenesis;, 2006
- Jolly RD, Walkley SU. Lysosomal storage diseases of animals: an essay in comparative pathology. Vet Pathol 1997; 34:527-548
- Miller AD, Zachary JF. Nervous system. In: Zachary JF, ed. Pathologic basis of veterinary disease, Elsevier, 2017, pp. 858-861.
- Sanders DN, Zeng R, Wenger DA, Johnson GS, Johnson GC, Decker JE, Katz ML, Platte SR, O'Brien DP: GM2 gangliosidosis associated with a HEXA missense mutation in Japanese Chin dogs: A potential model for Tay Sachs disease. Molecular Genetics and Metabolism 2013; 108(1):70-75.