Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 22
5 April, 2019
CASE II: 401324 (JPC 4103280)
Signalment: Seven-month-old female Limousine cross bovine (Bos taurus).
History: The animal was presented at the Scottish Centre for Production Animal Health and Food Safety (SCPAHFS) for not recovering after an episode of fog fever. Ultrasound examination revealed prominent hyperechoic appearance of the lungs. The animal was euthanased and submitted for post mortem examination.
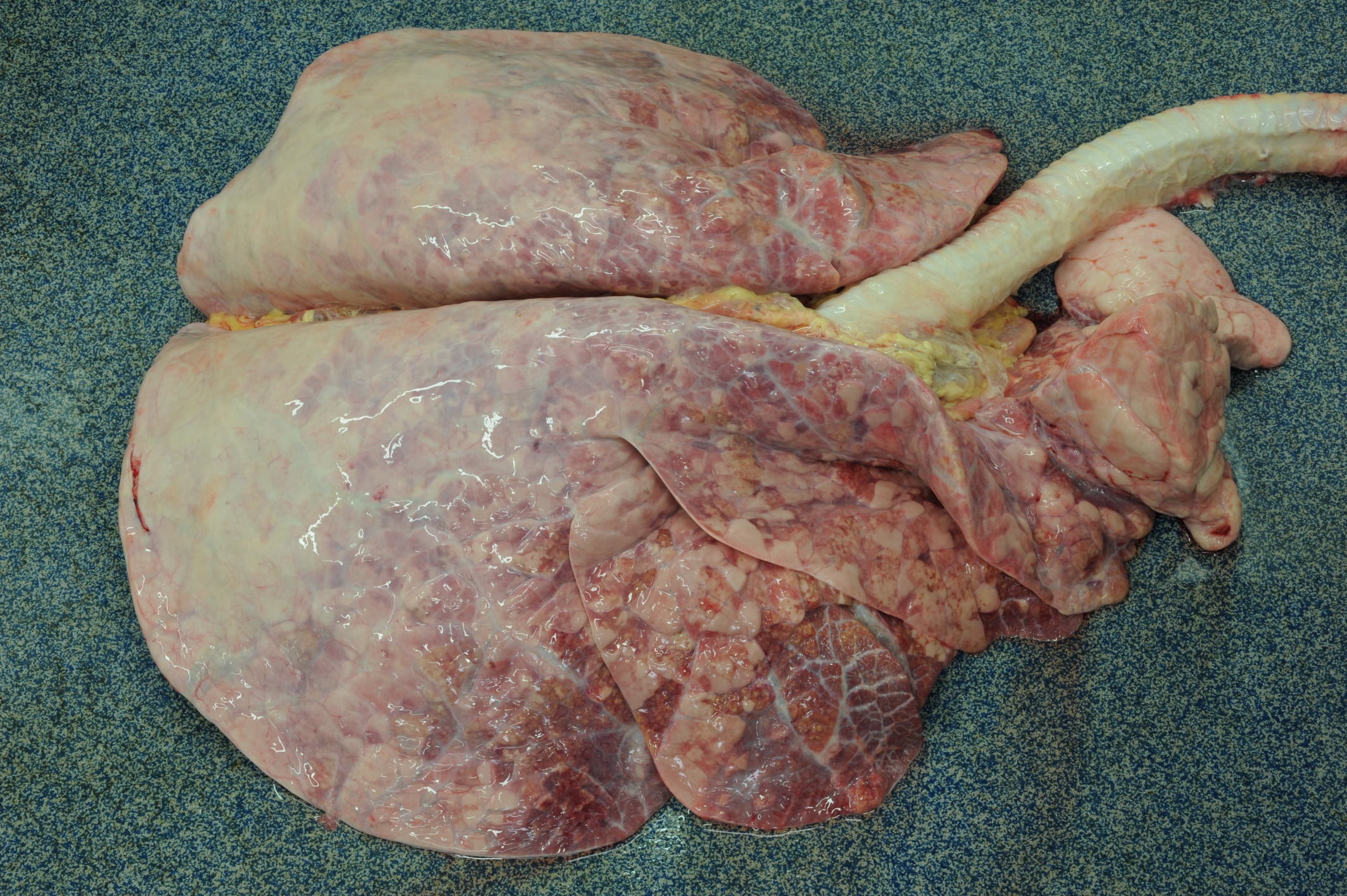
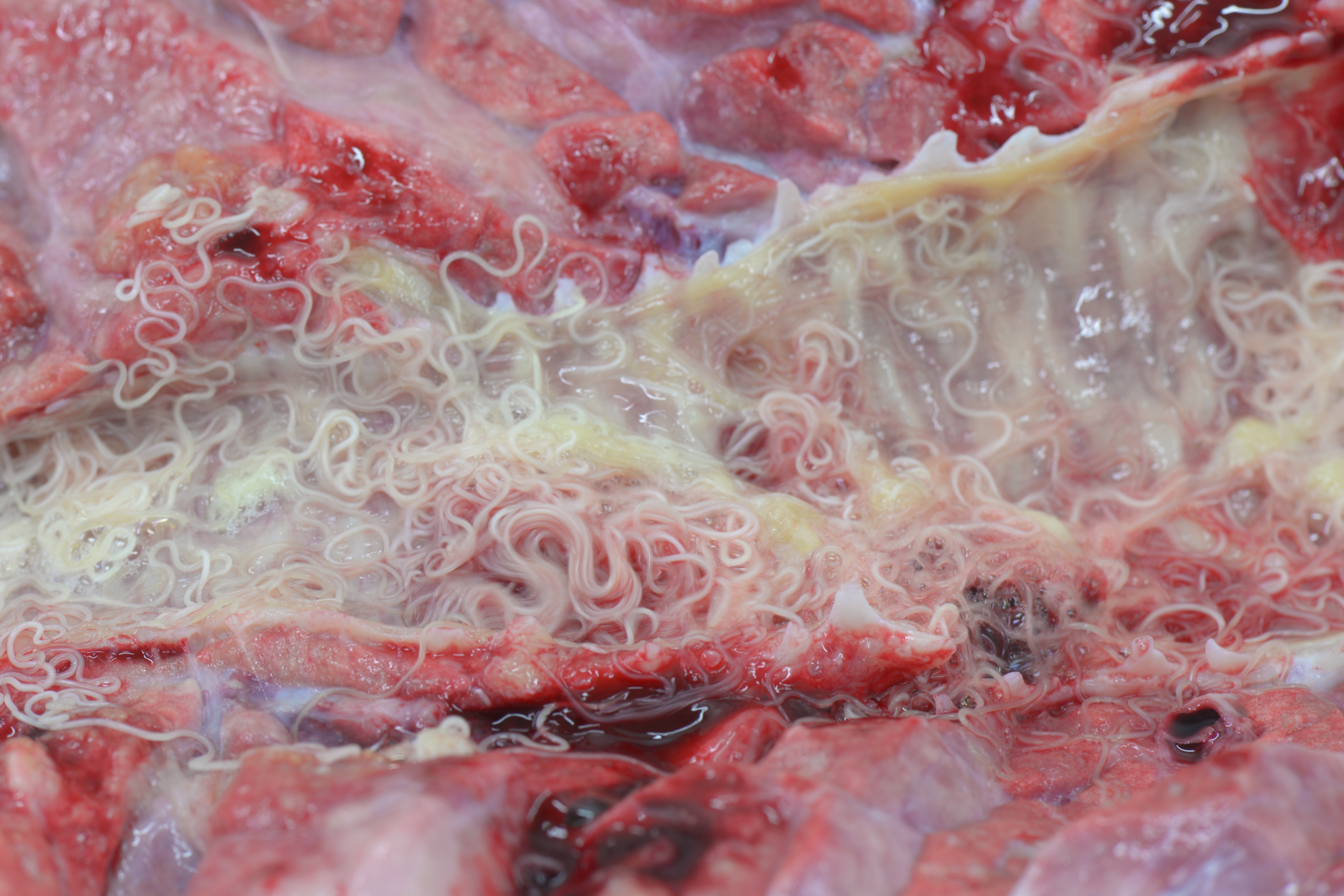
Gross Pathology: Caudal pulmonary lobes appear diffusely swollen; interlobular septa are prominently expanded by air. Cranioventral lobes exhibit multifocal to extensive and coalescing areas of reddish discoloration increased firmness; a yellowish mottling is noted on the cut surface of these consolidated areas. Very large numbers of long (4-5 cm in length) thin white round-worms are lying within trachea and bronchi admixed with frothy fluid and mucoid-purulent exudate, causing a partial obstruction of the lumen.
Liver exhibits multifocal small irregular areas (approximately 1x2 up to 2x3 cm) of yellow discoloration visible on the capsular surface close to the attachment of hepatic ligaments, and extending for approximately 0.5-1 cm into the parenchyma (tension lipidosis).
Kidneys display numerous bilateral small, round, whitish foci (1-3mm in diameter), that on cut surface correspond to pale streaks extending to corticomedullary junction.
A few scattered small (approximately 3-5 mm in diameter), dark shallow or slightly depressed lesions are evident on the abomasal mucosa.
Laboratory results: NA
Microscopic Description:
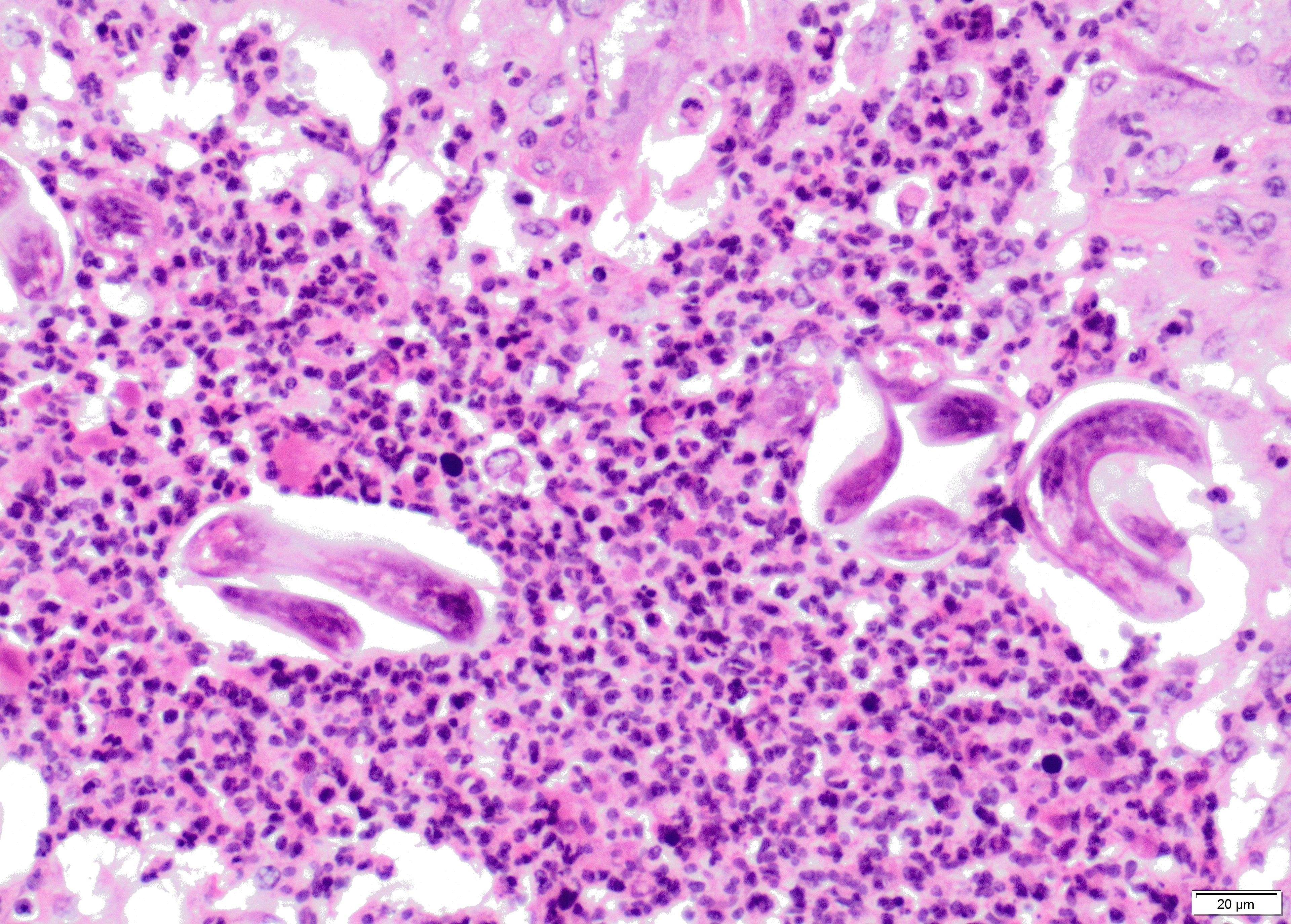
Lung: Up to 40% of the lung tissue is obliterated by extensive and coalescing areas of inflammation.
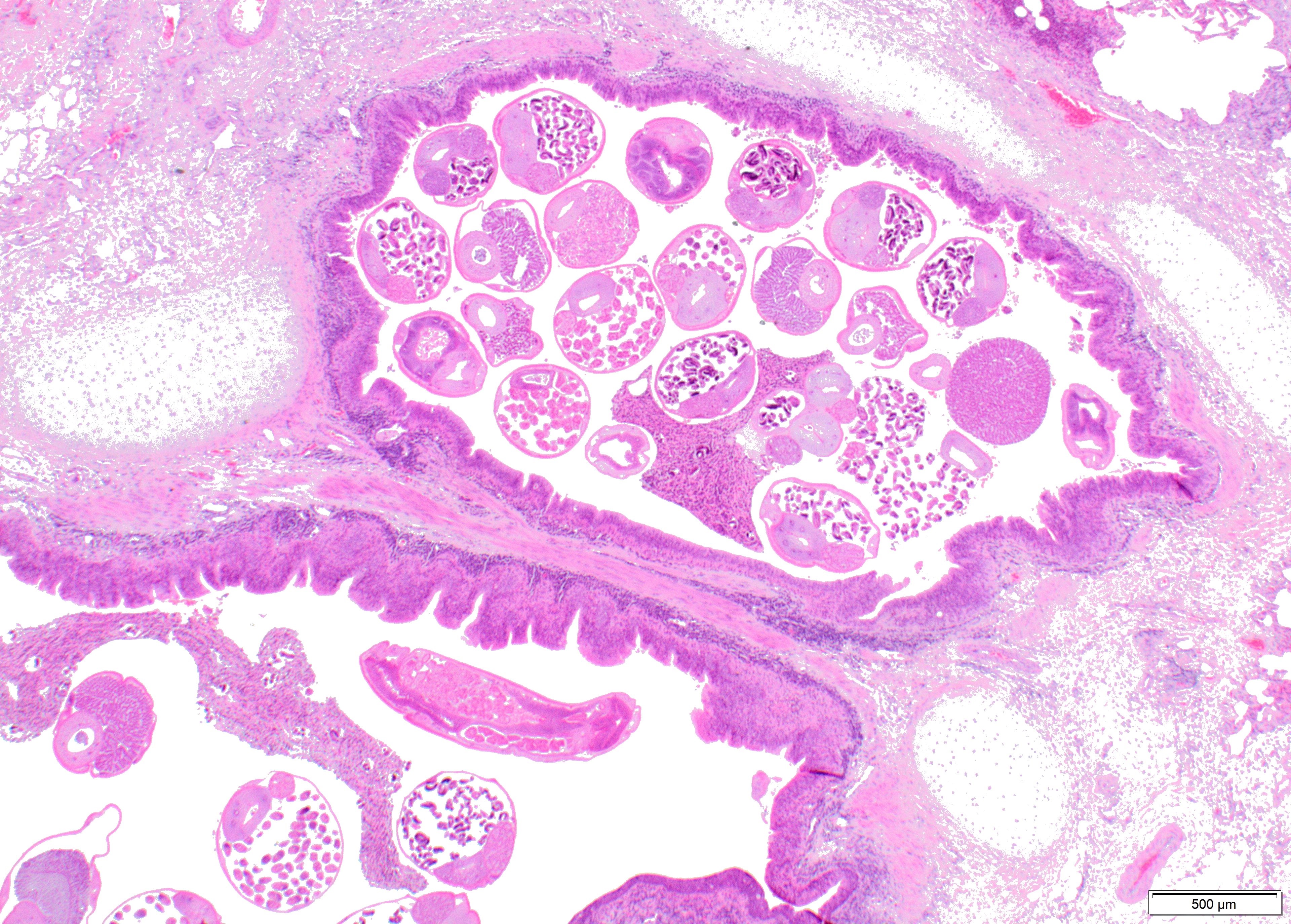
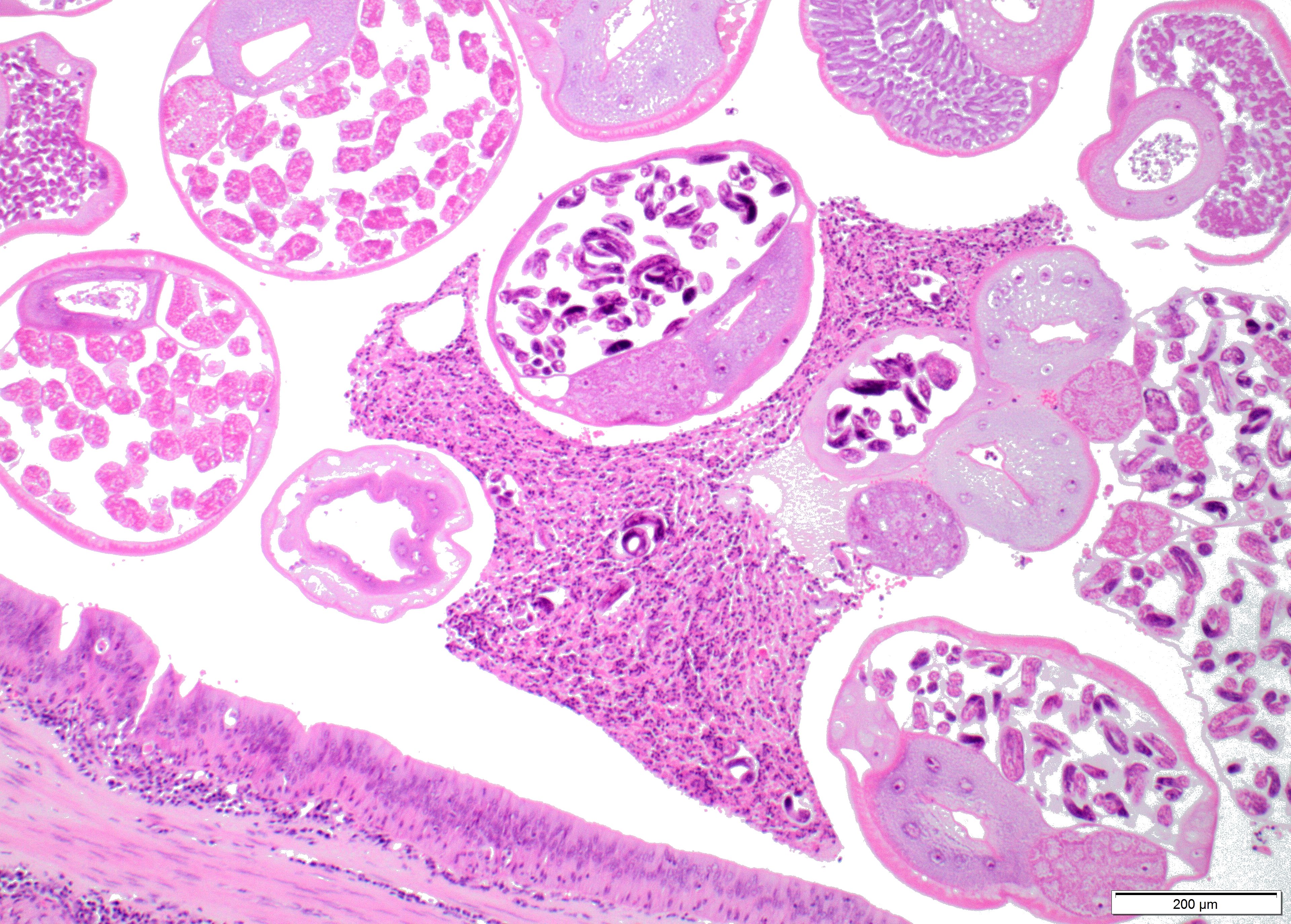
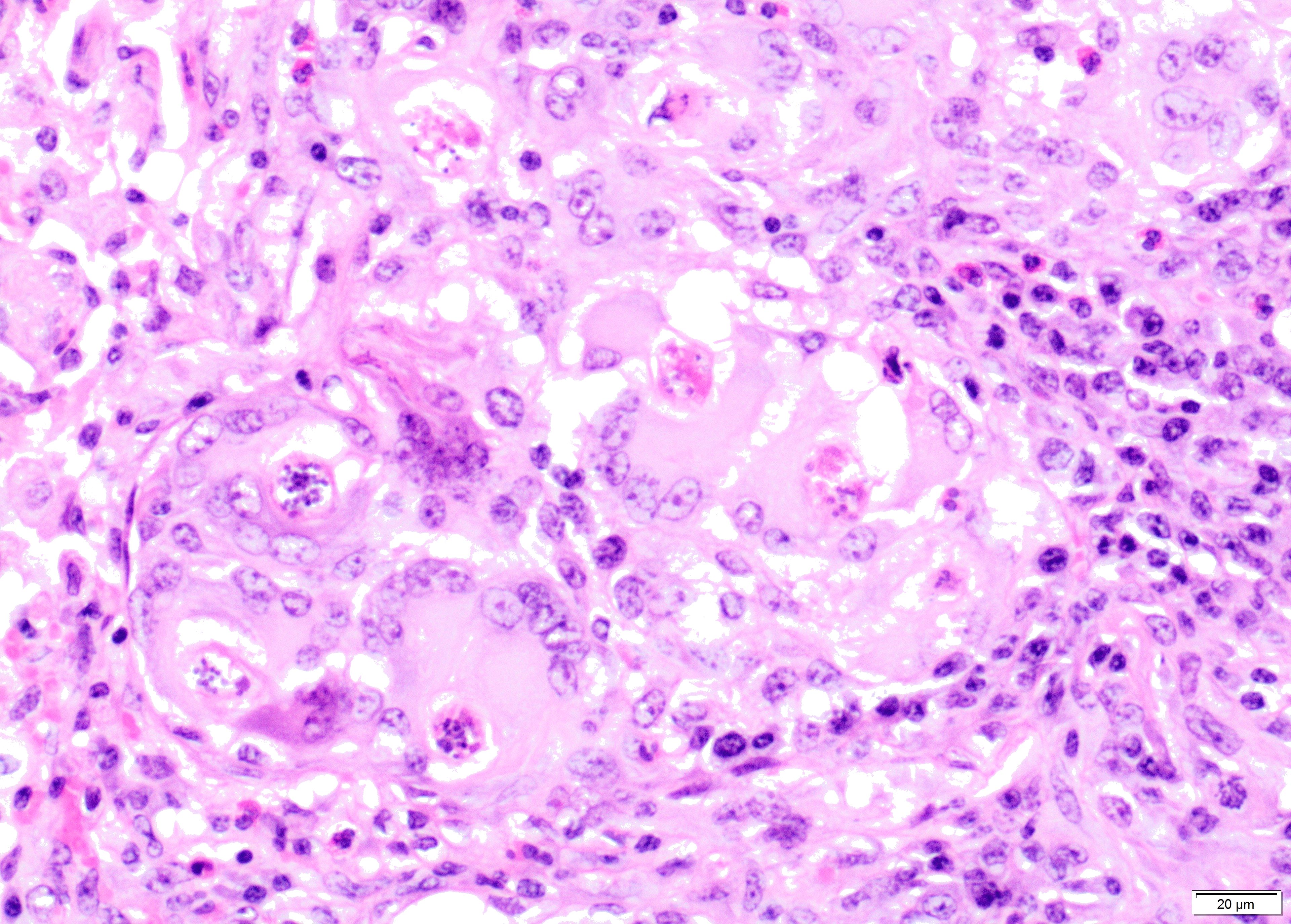
Within the lumen of medium sized to large bronchi there are numerous adult nematodes, variable numbers of sloughed degenerate/necrotic epithelial cells and scattered larvae. Parasites are approximately 350 µm in diameter, with an approximately 5 µm thick cuticle, platymyarian musculature, prominent lateral cords, a pseudocoelom, reproductive organs containing embryonated eggs or larvae, and a medium-sized intestine composed of multinucleated cells lined by short microvilli. The lumen of small bronchi and bronchioles is frequently plugged with large amounts of necrotic debris admixed with numerous degenerate neutrophils and eosinophils and large numbers of nematode larvae.
Alveolar spaces are filled with large numbers of granulocytes, including eosinophils and neutrophils, and variable numbers of macrophages, admixed with variable amounts of fibrillary to finely granular material and pale eosinophilic proteinaceous fluid (fibrin, cellular debris and edema) and numerous nematode larvae. Large numbers of plasma cells and lymphocytes expand the interstitium, along with large numbers of plump fibroblasts and increased amounts of collagen (interstitial fibrosis). Multifocal areas reveal ruptured alveolar septa with irregularly shaped clear areas resulting from coalescence of alveolar spaces (emphysema). In some areas, the inflammatory infiltrates obliterating the alveolar structures are characterised by large numbers of macrophages and variable numbers of multinucleate giant cells.
In some instances, bronchial and bronchiolar epithelium is hyperplastic (up to 6 cell layers), focal segmental squamous metaplasia is occasionally noted in larger bronchi.
Contributor’s Morphologic Diagnosis:
Lung: bronchopneumonia, eosinophilic and pyogranulomatous multifocal to coalescing, chronic, severe, with multifocal bronchiolar epithelial hyperplasia and squamous metaplasia, and adult and larval trichostrongyles.
Etiology: Dictyocaulus viviparus
Contributor’s Comment: Dictyocaulus viviparus is a member of the superfamily Trichostrongyloidea, family Dictyocaulidae.2 Trichostrongyles usually have platymyarian musculature. Characteristically, the external cuticle bears either evenly spaced or irregular longitudinal ridges. The buccal cavity in this group is not as pronounced as that of the true strongyles. Eggs of Dictyocaulus viviparus are embryonated (2).
The adult parasites are found in lower respiratory airways. Once delivered in the host tissue the eggs hatch rapidly. First stage larvae are coughed up, swallowed, and then passed in the feces. Further development into L2, and later L3, occurs in the feces on the pasture over the next 5-7 days and is strictly dependent on environmental conditions.2
A new host is infected by ingestion of third-stage larvae whilst grazing. These infective larvae penetrate the wall of the intestine, and migrate via the lymphatics to the mesenteric nodes. In the lymph nodes they form the forth-stage larvae and they enter the lungs about 7 days after the infection by means of lymph and blood. The final molt to the fifth stage occurs in the bronchioles, and adults develop in larger airways. Few egg-laying adults may persist in some animals and represent the source of pasture contamination in the following grazing season. Primary infections cause disease in calves during their first grazing season and clinical signs typically occur 3 to 5 months after exposure. Mortality rates depend on the degree of pasture contamination. Reinfection syndrome is observed when partially immune adult cattle on endemically affected farm have access to highly contaminated pastures. Some of the affected animals may develop fatal dyspnoea.2
Dictyocaulus viviparus is the only adult nematode known to infect the lung of the cattle and one of the most important parasites in cattle causing substantial economic losses. Current research is focused on the identification and characterization of genes associated with critical phases of the parasite life cycle and host-parasite interaction, with the purpose to exploit the gene products and related pathways as targets for vaccine design and/or drug development.4,5
Contributing Institution:
Division of Pathology, Public Health and Disease Investigation
Veterinary Diagnostic Services
School of Veterinary Medicine
College of Medical, Veterinary and Life Sciences
University of Glasgow (Garscube Campus)
464 Bearsden Road
Glasgow G61 1QH, Scotland
http://www.gla.ac.uk/schools/vet/
JPC Diagnosis: Lung: Bronchopneumonia, pyogranulomatous and eosinophilic, chronic, diffuse, severe, with alveolar and interlobular edema and emphysema and adult and larval metastrongyle nematodes.
JPC Comment:
While a number of lungworms may be present in larval form in the lungs of cattle, Dictyocaulus viviparous is the only one to establish a patent infection. Toxocara vitulorum and Ascaris suum larvae may migrate through the lungs as part of maturation and cause a transient pneumonitis, but adults will not be present in this location.1
In the prepatent phase (prior to maturation in to adults), gross lesions in the lungs are minimal, clinical signs coincide with penetration of larvae into alveoli where they incite an intense eosinophilic reaction which blocks small bronchi and bronchioles, resulting atelectasis and clinically cough and tachypnea.1
In the patent phase, there is usually bilaterally symmetric reddish of the caudoventral lobes with the presence of adult worms in the trachea and bronchi. In the postpatent phase, gross lesions are similar, but no adult worms or larvae are present. In this phase, up to 25% of severely infected animals may die as a result of a combination of severe pulmonary edema, extensive alveolar epithelial damage, and hyaline membrane formation. Histologically, animals experiencing a reinfection syndrome will develop greenish nodules up to 3-4 mm in diameter, which may be grossly visible underneath the pleura. The nodules are composed of parasitic debris and large numbers of eosinophils, macrophages, and multinucleated giant cells. In these cases, no edema or emphysema are present. More longstanding cases of reinfection syndrome may demonstrate formation of lymphoid nodules with germinal centers within the lungs. 1
References:
- Bowman DB, Zajac AM. Parasitic Bronchitis and Pneumonia. In: Smith B, ed. Large Animal Internal Medicine, 5th St. Louis MO: Elsevier, 2015:625-628
- Caswell JL, Williams KJ Respiratory System. In: Jubb, Kennedy, and Palmer’s. Pathology of Domestic Animals. 6th ed. St. Louis, MO: Elsevier; 2016: 554.
- Gardiner CH, Poynton SL. An Atlas of Metazoan Parasites in Animal Tissues. Washington DC: Armed Forces Institute of Pathology, American Registry of Pathology; 2006: 22, 29.
- McNulty S, Strübe C, Rosa BA, Martin JC, Tyagi R, Choi YJ, Wang Q, Hallsworth Pepin K, Zhang X, Ozersky P, Wilson RK, Sternberg PW, Gasser RB, Mitreva M. Dictyocaulus viviparous genome, variome and transcriptome elucidate lungworm biology and support future intervention. Sci Rep. 2016; 6: 20316.
- Strube C, Buschbaum S, Schnieder T. Genes of the bovine lungworm Dictyocaulus viviparus associated with transition from pasture to parasitism. Infect Genet Evol. 2012; 12: 1178-1188.