Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 9
November 1st, 2017
CASE II: DX17-0022 (JPC 4101486).
Signalment: Unknown age, female, NSG mouse (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ), Mus musculus, mouse.
History: The mouse was part of a tumor xenograft study and was removed based on predetermined study endpoints for the pathology evaluation.
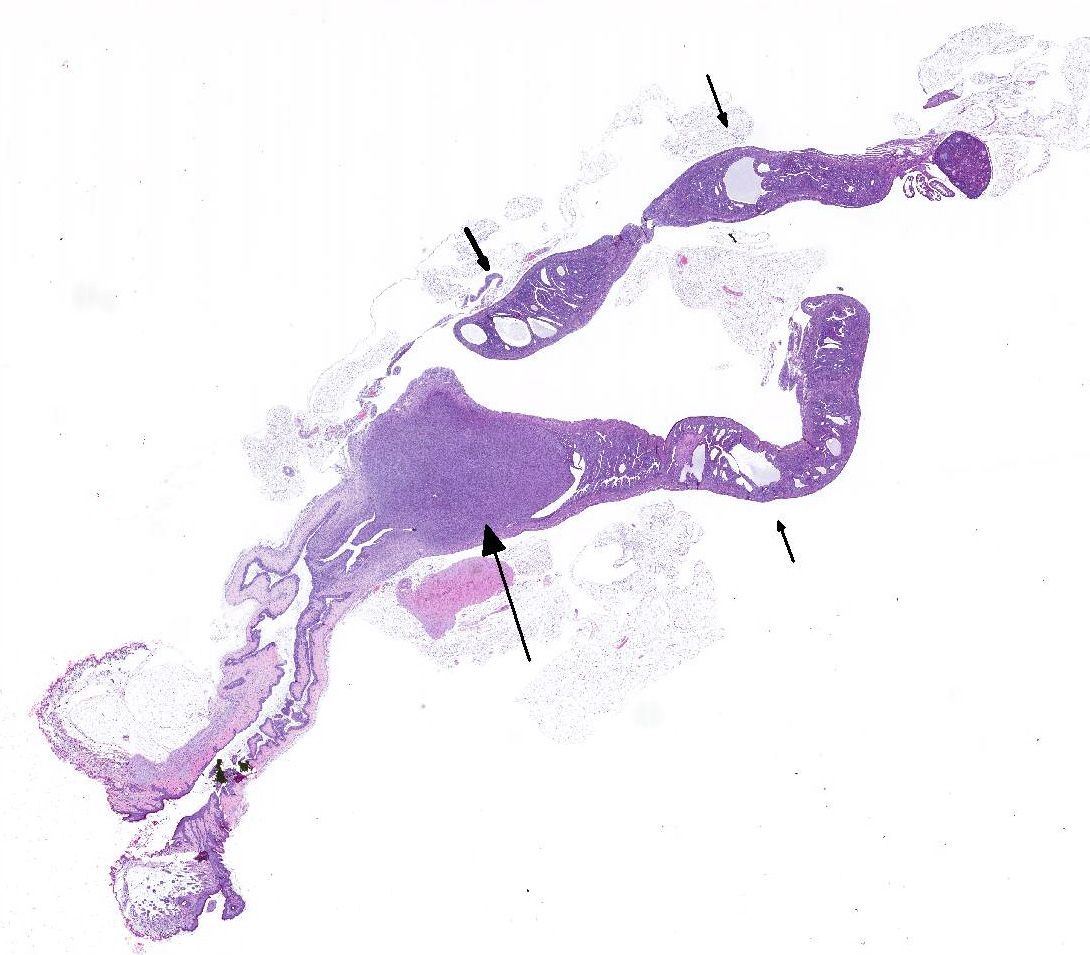
Gross Pathology: A small-sized, white firm single mass was observed within the wall of the horn of the uterus. No other gross lesions were noted at the time of necropsy.
Laboratory results:
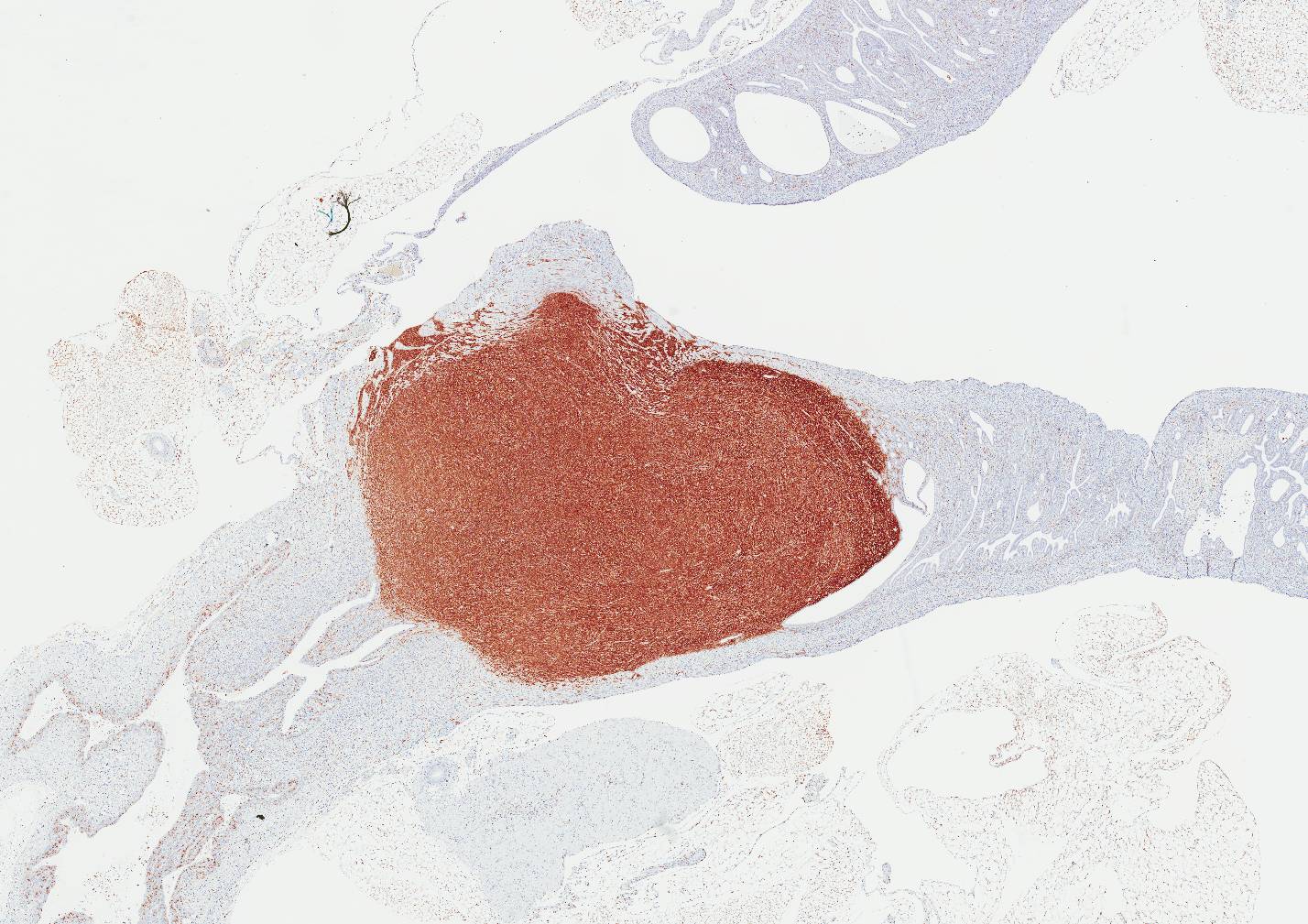
Immunohistochemistry was performed on the mass:
MAC2: Diffuse, membranous to cytoplasmic labeling of >95% of tumor cells
CD68: Diffuse, membranous to cytoplasmic labeling of >95% of tumor cells
Lysozyme: Diffuse, cytoplasmic labeling of >95% of tumor cells
S100: Negative diffuse, cytoplasmic labeling with blush intensity that is consistent with background; Scattered cells representing neutrophils are positively labeled
Langerin: Negative; Positive control, Haired skin: Scattered immune cells representing dendritic cells in the epidermis have positive cytoplasmic labeling
CD163: Negative; Scattered cells representing tumor infiltrating macrophages are positively labeled
CD45R/B220: Negative
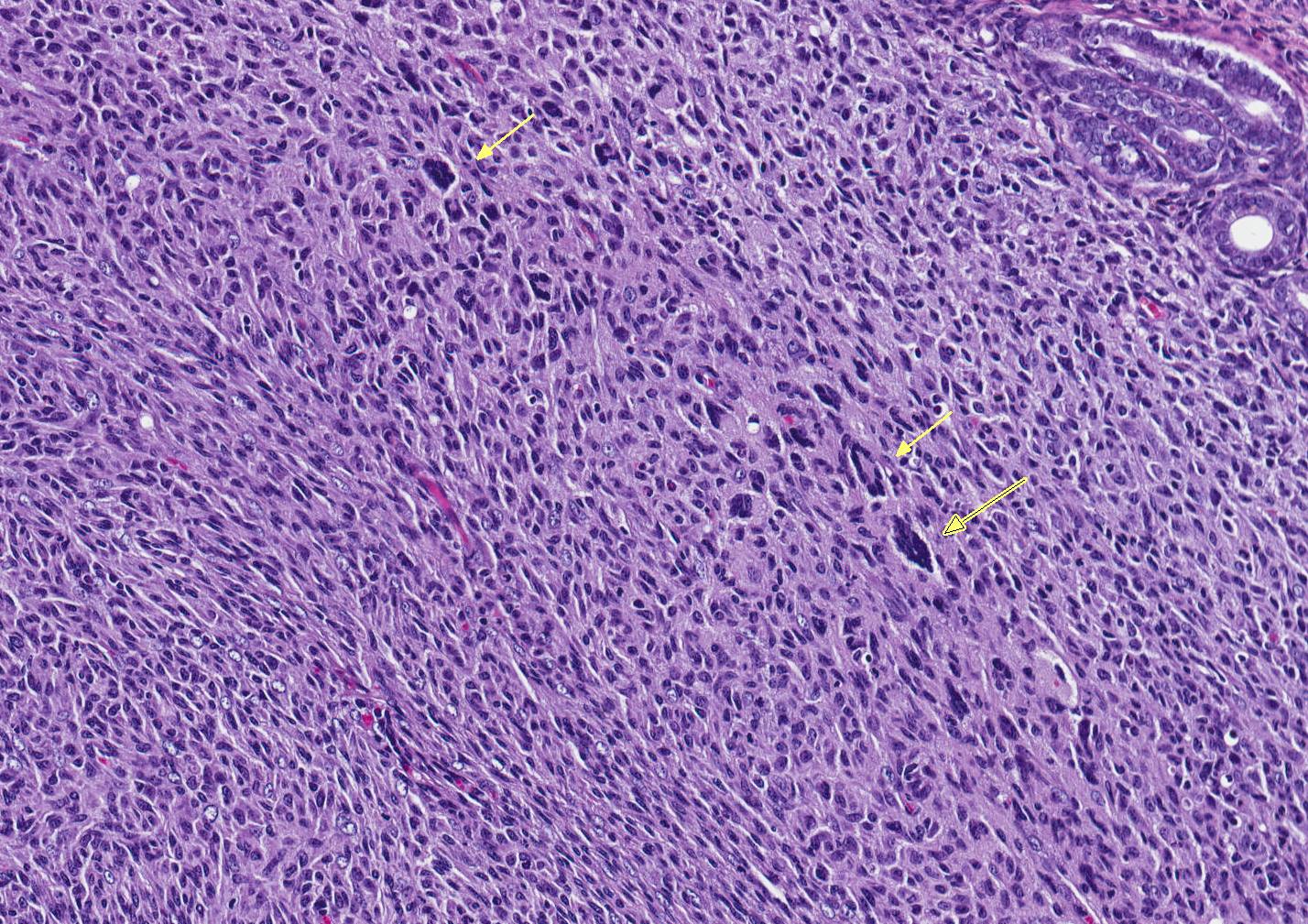
Microscopic Description: An unencapsulated, nodular mass expands a portion of the endometrium. Neoplastic cells are arranged in tight bundles and streams that are situated within a collagenous stroma. Neoplastic cells are spindle-shaped, have moderate amounts of eosinophilic cytoplasmic and have fusiform, reticulated nuclei with 1-2 prominent nucleoli. There is moderate anisocytosis and anisokaryosis. Mitoses number from 0-1 per 400x/HPF. Low numbers of neutrophils are present throughout the neoplasm. Metastatic lesions are not observed.
Contributors Morphologic Diagnosis: Uterus, endometrium: Histiocytic sarcoma.
Contributors Comment: Histiocytic sarcomas arising in the uterus of laboratory mice are well-described, vary greatly in morphologic patterning, occur infrequently (~12%) and often involve other organs.4,6 The putative cells of origin for histiocytic sarcomas for both humans and animals are considered to be CD34+ myeloid dendritic cells that have immunophenotypic characteristics of mature tissue histiocytes and possible shared clonal origins as other leukemias.3
In the mouse, several markers may be used to distinguish histiocytic sarcomas from other hematopoietic tumors, especially histiocyte-rich neoplasms. The markers that have shown good reproducibility by immunohistochemistry (IHC) include: CD163, IBA1, CD68, F4/80, lysozyme and MAC2. The latter five markers are reported to be expressed by histiocytic sarcomas of variable frequency. Histiocytic sarcomas in mice and humans are frequently reported to be negative for the following lineage markers, (where applicable by species): Langerhans cell markers (e.g., Langerin/CD207, CD1A), follicular dendritic cells (CD21/CD35), T-cell related markers (e.g., CD3), common myeloid cell markers (e.g., MPO), melanocytic markers and epithelial cell markers.7,8,9 The lack of CD163 expression suggests this sarcoma is not derived from tissue macrophages. However, the sarcoma expresses combinations of common histiocyte markers that may include both Langerhans and dendritic cell populations and is most consistent with tumor cells that have originated from a common bone marrow derived histiocytic progenitor.
NSG mice are deficient in mature lymphocytes and NK cells, survive beyond 16 months of age, and are relatively resistant to lymphoma development making this strain useful for long-term engraftment studies using human cells.11 Because this strain of mouse has been developed relatively recently the incidence of spontaneously arising tumors is only starting to be documented. At least one MAC2 positive histiocytic sarcoma with multi-organ involvement has been reported.10 Because this strain is used for engraftment studies understanding the incidence of spontaneously arising tumors, especially involving the neoplastic transformation of hematopoietic stem/progenitor cell populations is necessary for determining study related outcomes.
JPC Diagnosis: 1. Uterine body: Histiocytic sarcoma, NSG mouse (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) (Mus musculus), mouse.
2. Uterus, endometrium: Hyperplasia, cystic, diffuse, moderate.
Conference Comment: Histiocytic sarcomas (HS) arise from cells of the mononuclear-phagocytic lineage, and therefore, may be arise in any tissue of the body. Gross findings generally include enlargement of the spleen with nodular lesions in any number of additional organs (liver, uterus, vagina, kidney, lung ovaries). Neoplastic cells are large within irregular deeply basophilic nuclei, pale fibrillar cytoplasm, and fairly distinct cell borders. Often giant nuclei and multinucleated cells are present. Cells vary in roundness and may appear somewhat elongate, forming interlacing streams and bundles palisading along pre-existing stroma, particularly when located within the uterine wall.1 These neoplasms are often mistaken for malignant schwannomas. In the liver, HS cells are often seen phagocytizing red blood cells (erythrophagocytosis). When HS are in lymph nodes, they are difficult to differentiate from histiocyte-associated diffuse large B-cell lymphomas and immunohistochemistry is required for definitive diagnosis. There are many markers for cells of dendritic origin (listed above) but CD18 positive membrane staining of suspected cells is diagnostic for HS.2 Certain strains of mice (B6 and SJL) and rats (SD) are prone to HS.
An interesting associated lesion in mice is hyaline droplets within the cytoplasm of renal tubular epithelial cells which represent lysozyme released from apoptotic neoplastic histiocytes.5 In hamsters, there has been an association noted between development of hepatic HS and Helicobacter spp. related hepatitis.1
In dogs, HS are the most commonly occurring joint tumors with many breed predispositions (Bernese Mountain Dogs, Rottweilers, Bullmastiffs, golden Retrievers, Labrador Retreivers, and Flat-Coated Retrievers) as well as environmental factors that predispose such as joint damage from arthritis or ruptured cruciate ligaments. HS in dogs has a different gross appearance and is more lobulated, filling the joint cavity, and infiltrating adjacent soft tissue. Microscopically, cells have a more vacuolated cytoplasm which has led to mistaken identification as liposarcomatous neoplastic cells. Cells have a similar appearance as was previously described for rodents, but joint HS have a more favorable prognosis than those originating in the spleen.2
Attendees noted the cystic changes in the endometrium, and while most attendees attributed them to cystic endometrial hyperplasia - the moderator suggested that these may also simply be present as a result of compression from the mass.
The moderator also noted that NSG mice are immune-deficient, and similar to nude mice, are susceptible to hyperkeratotic skin diseases caused by Corynebacterium, Staphylococcus aureus or S. xylosus (ulcerative dermatitis). C. bovis grows within keratin due to its lipophilic nature and can persist in the environment within keratin flakes. Microscopically, they have diffuse hyperkeratosis with little dermal inflammation and gram-positive rods can be easily identified among the layers of keratin. S. aureus and S. xylosus are both commensal bacteria and are common in the environment. In immunosuppressed mice and rats, they can colonize the outer dermis and produce numerous proteins (hemolysins, nucleases, proteases, lipases, hyaluronidase, and collagenase) and exotoxins (exfoliative toxins, leukocidin, superantigens) which damage the skin and produce burn-like features.1
St. Jude Childrens Research Hospital
Department of Pathology
MS 250, Room 5031
262 Danny Thomas Place
Memphis, TN, 38105-3678
https://www.stjude.org/research/departments-divisions/pathology.html
References:
1. Barthold SW, Griffey SM, Percy DM. Pathology of Laboratory Rodents and Rabbits. Ames, IA: John Wiley & Sons, Inc.; 2016:67-70, 103, 110-111, 167-168, 183.
2. Craig LE, Dittmer KE, Thompson KG. Bones and joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol. 1. 6th ed. St. Louis, MO: Elsevier; 2016:159-160.
3. Feldman AL, Minniti C, Santi M, Downing JR, Raffeld M, Jaffe ES. Histiocytic sarcoma after acute lymphoblastic leukaemia: a common clonal origin. The Lancet Oncology. 2004; 5(4): 248-250.
4. Hao X, Fredrickson TN, Chattopadhyay SK, Han W, Qi CF, Wang Z, et al. The histopathologic and molecular basis for the diagnosis of histiocytic sarcoma and histiocyteassociated lymphoma of mice. Veterinary Pathology. 2010; 47(3): 434-445.
5. Hard GC, Snowden RT. Hyaline droplet accumulation in rodent kidney proximal tubules: an association with histiocytic sarcoma. Toxicologic Pathology. 1991;19(2):88-97.
6. Lacroix-Triki M, Lacoste-Collin L, Jozan S, Charlet J-P, Caratero C, Courtade M. Histiocytic sarcoma in C57BL/6J female mice is associated with liver hematopoiesis: review of 41 cases. Toxicologic Pathology. 2003; 31(3): 304-309.
7. Pileri SA, Grogan TM, Harris NL, Banks P, Campo E, Chan JKC, et al. Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology. 2002; 41(1): 1-29.
8. Rehg JE, Bush D, Ward JM: The utility of immunohistochemistry for the identification of hematopoietic and lymphoid cells in normal tissues and interpretation of proliferative and inflammatory lesions of mice and rats. Toxicologic Pathology. 2012; 40(2): 345-374.
9. Rehg JE, Ward JM. Applications of immunohistochemistry-toxicologic pathology of the hematolymphoid system, In: Parker G, ed. Immunopathology in Toxicology and Drug Development: Volume 1, Immunobiology, Investigative Techniques, and Special Studies. 1st ed. Cham, Switzerland: Humana Press; 2017: 489-551.
10. Santagostino SF, Arbona RJR, Nashat MA, White JR: Pathology of aging in NOD scid gamma female mice. Veterinary Pathology. 2017; 54(5): 855-869.
11. Shultz LD, Lyons BL, Burzenski LM, Gott B et al.: Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R?null mice engrafted with mobilized human hemopoietic stem cells. The Journal of Immunology. 2005; 174(10): 6477-6489.