Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 15
January 17th, 2018
CASE I: S16-1796 (JPC 4101316).
Signalment: 21-week-old, male, Eagle owl, Bubo bubo, avian.
History: The animal’s general condition was reduced (sunken eyes, bristling of the feathers, anorexia) and got constantly worse over 3 days. The animal died subsequently despite treatment with glucose and activated charcoal. The animal originated from a falconry, was kept in an aviary with the possibility of free flight and was mostly fed on chicken and pigeons.
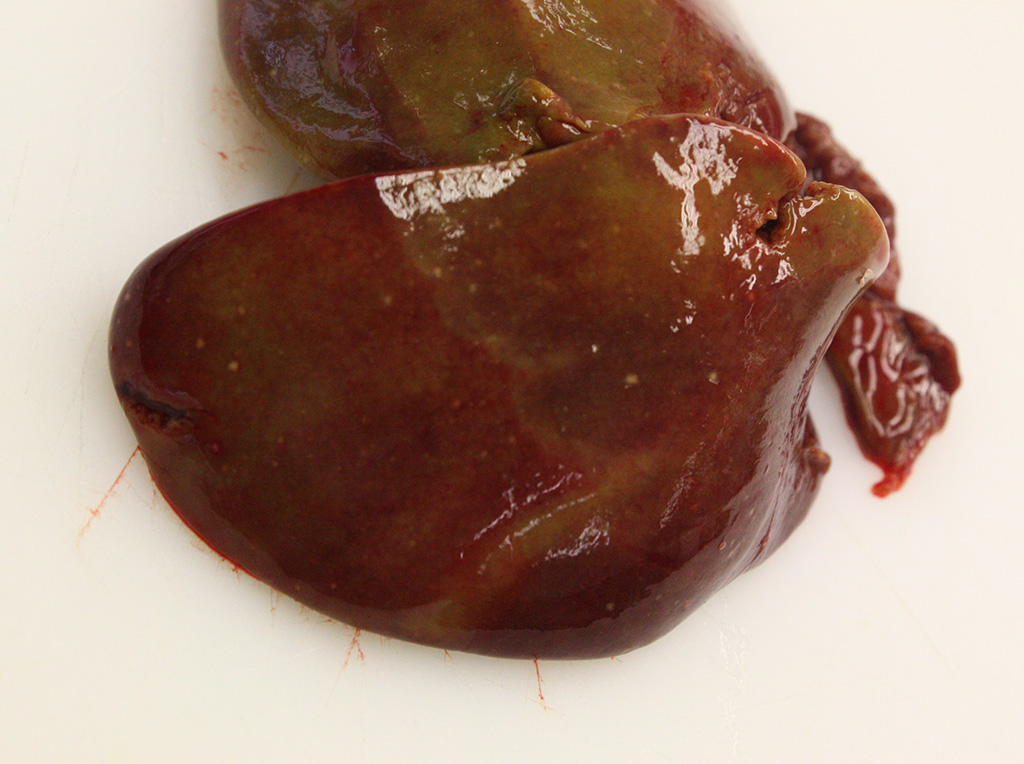
Gross Pathology: The animal was in moderate body condition. The liver had a light brown to beige color. On the surface, randomly distributed on all lobes, small (<1 mm in diameter), sharply demarcated, whitish-yellowish foci were found. On the mucosa of the small intestine, randomly distributed, round, 2 mm in diameter, well demarcated, whitish-yellowish foci were detected. The spleen was considerably swollen and showed a dark red to light violet color. Round, whitish-yellowish foci were also detected on the surface of the spleen.
Laboratory results:
Bacteriological examination of liver (culture): Negative.
Parasitological examination of feces: Negative.
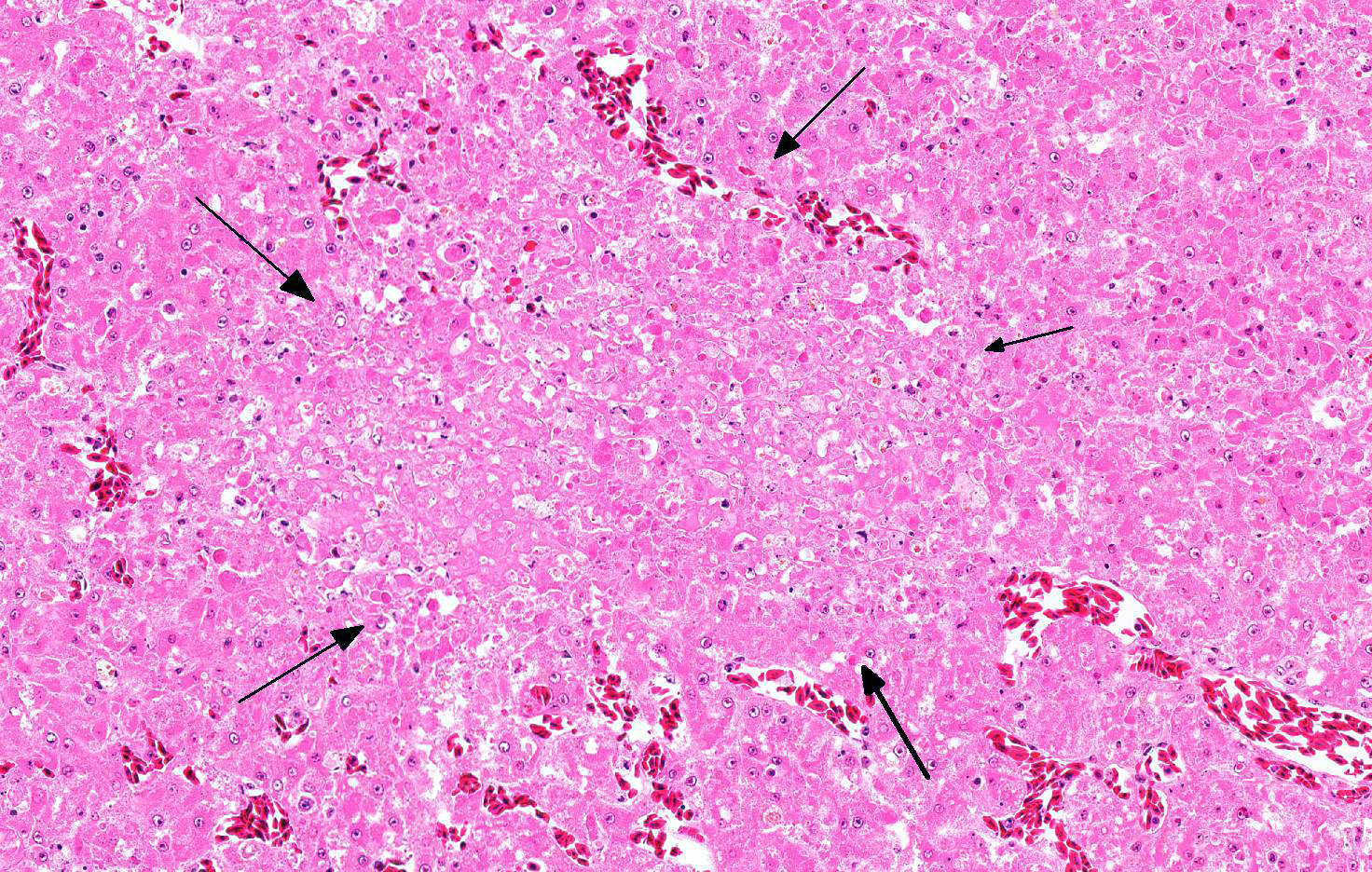
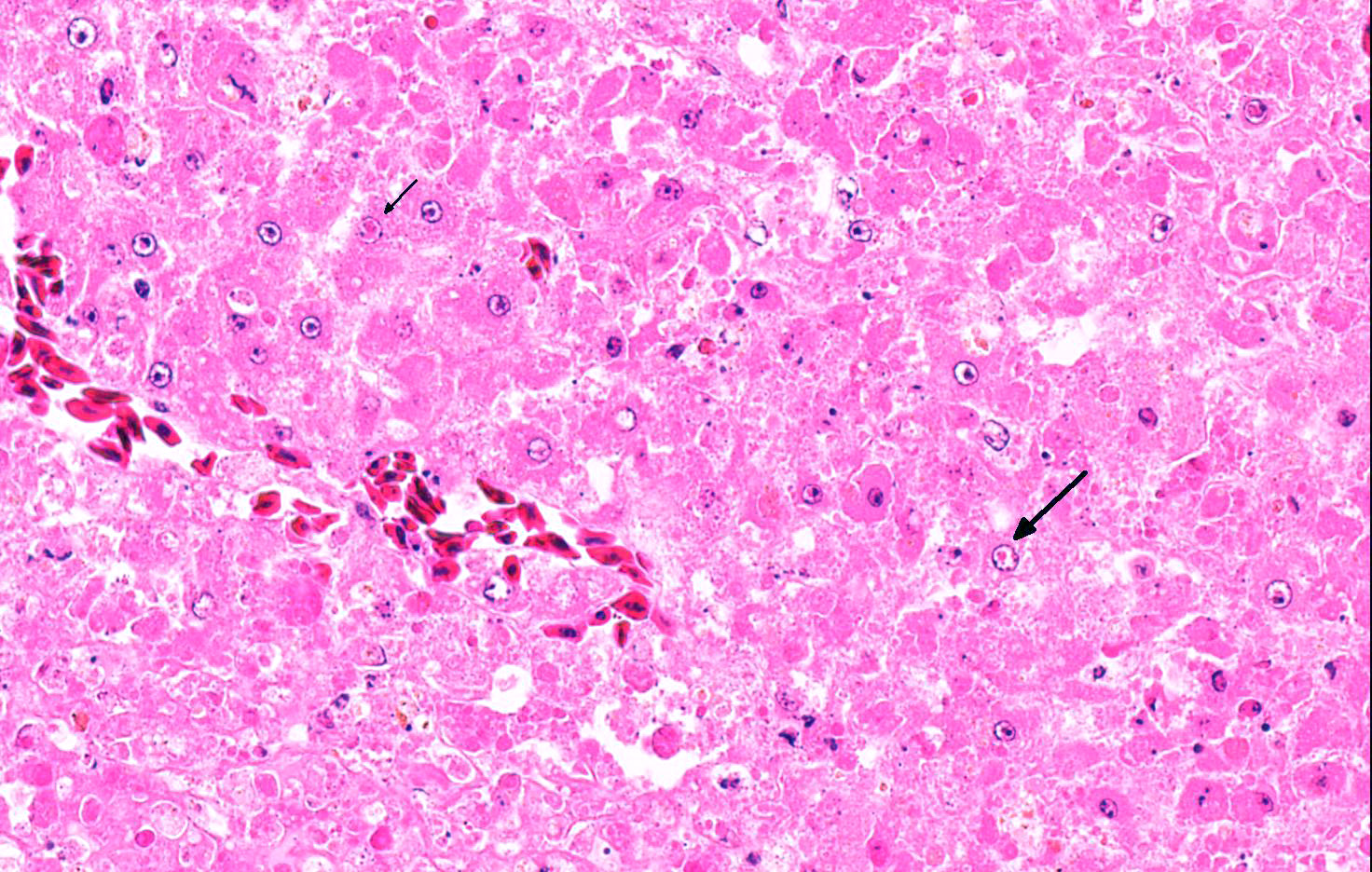
Microscopic Description: Liver: Approximately 70% of the liver had multifocal to coalescing randomly distributed foci of coagulative necrosis, characterized by hypereosinophilic hepatocytes with karyopyknosis and karyorrhexis. The inflammatory response surrounding foci of necrosis was minimal and consisted of a few macrophages. Intranuclear eosinophilic inclusion bodies and chromatin margination occurred in hepatocytes bordering the necrotic areas.
Contributor’s Morphologic Diagnosis:
Liver: Hepatitis, multifocal, acute, necrotizing, severe with intranuclear eosinophilic inclusion bodies within hepatocytes.
Contributor’s Etiologic Diagnosis: Columbid herpesvirus 1
Contributor’s Comment: Gross and histologic lesions in this owl were highly suspicious of an infection with herpesvirus and this was confirmed by the detection of the characteristic intranuclear inclusion bodies in hepatocytes bordering the necrotic foci. In addition to necrotizing hepatitis, necrotizing splenitis and necrotizing enteritis was seen in this animal. The owl has been kept in an aviary and fed on chicken and pigeons, the latter being the most likely source of infection; pigeons are often subclinically infected with columbid Herpesvirus 1 that can cause disease in owls.8
In 1932 in North America herpesvirus was first reported in owls and subsequently in prairie falcons (Falco mexicanus), American kestrels (Falco sparverius) and the peregrine falcon (Falco peregrinus).8 Originally herpesvirus isolates from falcons and owls have been considered as distinct viruses, namely falconid herpesvirus 1 (FHV-1) and strigid herpesvirus 1 (StHV-1). However, serologic studies showed cross-reactivity among FHV-1, StHV-1 and columbid herpesvirus-1 (CoHV-1) as well as that CoHV-1 and FHV-1 are indistinguishable and finally PCR studies confirmed that inclusion body hepatitis in falcons and owls is caused by columbid herpesvirus-1.3
In owls, falcons, and eagles, the disease is known as hepatosplenitis. The disease usually has an acute to subacute course. Clinical signs are nonspecific, including weakness, anorexia and depression.7 Characteristic gross lesions in falcons are small white hepatic and splenic foci representing necrosis and similar foci in the bone marrow of falcons.3 Histologically, areas of coagulative necrosis are detectable in the spleen, liver and bone marrow, usually with only little associated inflammation.3 Intranuclear eosinophilic inclusion bodies within hepatocytes or macrophages bordering the necrotic areas can frequently be detected.3 Lesions caused by CoHV-1 are not restricted to the liver and spleen; often the small intestine and the kidney are also involved.6 The progression of the disease is associated with rapid virus multiplication and organ dysfunction. Most reported cases of disease in falcons and owls involve prior documented or possible ingestion of pigeons or by direct contact with the infected bird.4,8
In pigeons, Hhrpesvirus-induced disease was first described in Rock Pigeons (Columbia livia) and was called “inclusion body disease” or “inclusion body hepatitis”.3 Herpesvirus is prevalent in the pigeon population and has little to no effect on the health of this species. Pigeons, especially those kept in captivity, are common subclinical carriers of the virus. However, disease occurs in squabs aged ten to sixteen weeks. Clinical signs such as depression, anorexia, conjunctivitis, oral and pharyngeal ulceration, dyspnea, and diarrhea with a duration of a few hours to as long as one week are reported, but not always present. Histological lesions include hepatic and splenic necrosis as reported in owls and falcons. In addition upper respiratory tract inflammation with ulceration and upper gastrointestinal tract inflammation with ulceration is described. Epithelial and parenchymal cells bordering the necrotic areas contain eosinophilic intranuclear inclusions.3,4
The disease is transmitted to squads by chronically infected male and female breeding pigeons when feeding regurgitated crop milk during the first weeks of life of the squabs.5 Contact during courtship, preening and mutual feeding of adult pairs during mating does not result in virus transmission. Ingested virus replicates in the oropharynx region, followed by short-term viremia and virus multiplication in all internal organs. Squabs show severe epithelial lesions in the pharynx, esophagus and crop. Infection by pigeon herpesvirus only rarely results in clinically overt forms of disease in adults and adult pigeons usually do not present any clinical signs except depression, anorexia or conjunctivitis.5 Exact data on the prevalence of herpesvirus disease in pigeons is not available. Numerous reports provide evidence for the presence of the pigeon herpesvirus in all European countries and many pigeon lofts. Pigeon herpesvirus has been detected in all breeds of domestic pigeons (Columba livia f. domestica), feral pigeons, and other members of the family Columbidae.5
JPC Diagnosis: Liver: Hepatitis, necrotizing, random, multifocal to coalescing, moderate with intranuclear eosinophilic viral inclusion bodies, Eagle owl (Bubo bubo), avian.
Conference Comment: Hepatosplenitis caused by herpesvirus in owls (OHV) and falcons (FHV) are genetically similar and are both pathogenic for owls, ring-necked doves (Streptopelia sp.) and kestrels (Eurasian and American).2 In a comprehensive review of OHV, Drs. Burtscher and Sibalin reviewed the wide spectrum of hosts affected and identified the tawny owl and barn owl as being resistant.1 Herpesviral disease in birds of prey is often Peracute, resulting in rapid fatalities. However, subclinical cases do occur and are characterized by lethargy, anorexia, diarrhea, and a progressive leukopenia. Gross lesions include hepatomegaly and splenomegaly with pharyngeal and intestinal lesions in owls. Microscopically, there is hepatic necrosis with prominent intranuclear inclusion bodies most prominent at the edge of the necrotic tissue.2
The virus is often spread through infected pigeons,as in this case, and is a risk to both captive and free-living birds of prey around the world. Diagnosis is usually based on gross lesions and identification of the characteristic eosinophilic intranuclear inclusion bodies microscopically which are pathognomonic for this disease.9 There is no successful treatment for viral hepatitis and most often infected birds are culled to prevent spread of the disease. In comparison to psittacine herpesviruses, falcon and owl herpesviruses are more resistant to chemical disinfectants.2
The main differential for liver and intestinal lesions in raptors with prominent intranuclear inclusion bodies would be adenovirus. However, most of these animals are subclinically infected and diagnosis is often made at necropsy.2
Conference participants noted that there was some slide variation with mixed colonies of bacteria in areas of necrosis on some slides (presumed postmortem overgrowth) and intranuclear inclusion bodies in bile duct epithelial cells.
Contributing Institution:
Institute of Veterinary Pathology
Vetsuisse-Faculty (University of Zurich)
Winterthurerstrasse 268, CH-8057 Zurich
Fax number +41 44 635 89 34
www.vetpathology.uzh.ch
References:
- Burtscher H, Sibalin M. Herpesvirus stringis: host spectrum and distribution in infected owls. J Wildl Dis. 1975;11(2):164-169.
- Cooper JE. Infectious diseases, excluding macroparasites. In: Birds of Prey Health & Disease. 3rd Oxford, UK: Blackwell Science Ltd.; 2002:101-102.
- Gailbreath K, Oaks L. Herpesviral inclusion body disease in owls and falcons is caused by the pigeon herpesvirus (Columbid herpesvirus-1). J Wildlife Dis. 2008;44:427-433.
- Kaleta E, Docherty D. Avian herpesviruses. In: Thomas N, Hunter D, eds. Infectious Disease of Wild Birds. Ames, IA: Blackwell Publishing; 2007:63-86.
- Kaleta EF. Herpesviruses of birds- a review. Avian Pathology. 1990;19(2):193-211.
- Mozos E, Hervas J, Moyano T, Diaz J, Gomez-Villamandos JC. Inclusion body disease in a peregrine falcon (Falco peregrinus): histological and ultrastructural study. Avian Pathology. 1994;23(1):175-181.
- Pinkerton M, Wellehan J, Johnson A, Childress A, Fitzgerard S, Kinsel M. Columbid herpesvirus-1 in two Cooper’s hawks (Accipiter cooperii) with fatal inclusion body disease. J Wildl Dis. 2008; 44:622-628.
- Rose N, Warren Al, Whiteside D, Bidulka J, Robinson JH, Illanes O, Brookfield C. Columbid herpesvirus-1 mortality in great horned owls (Bubo virginianus) from Calgary, Alberta. Can Cet J. 2012;53:265-268.
- Scott DE. Infectious diseases. In: Raptor Medicine, Surgery, and Rehabilitation. 2nd Oxfordshire, UK: CABI; 2017:117-118.