Signalment:
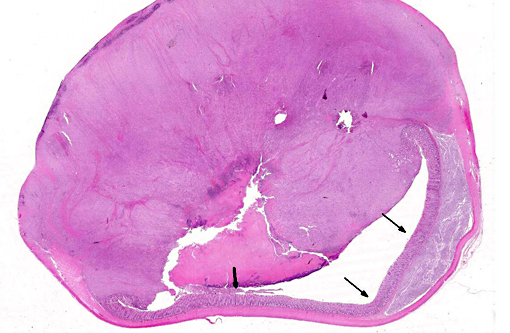
Gross Description:
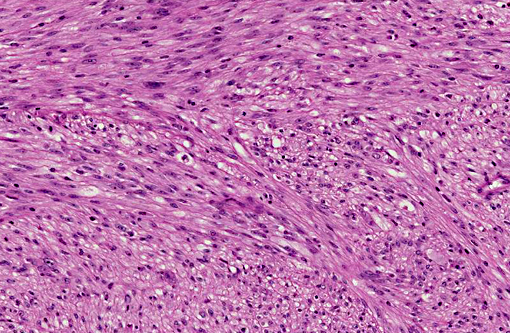
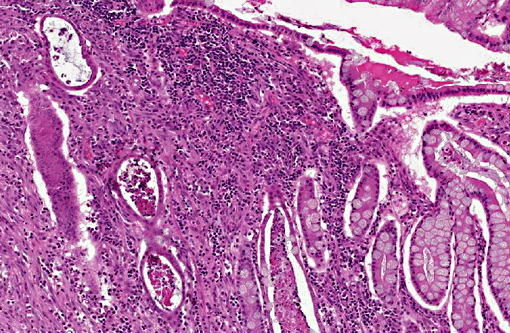
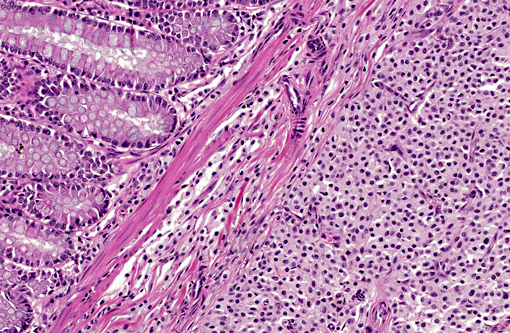
Histopathologic Description:
The neoplastic cells did not express alpha smooth muscle actin. Additional tissue sections were immunohistochemically stained for CD117 (c-KIT) and revealed scattered neoplastic cells exhibiting weak to moderate CD117 (c-KIT) positivity.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
GISTs are described as a distinctive group of primary mesenchymal gastrointestinal tumors assumed to be derived from the interstitial cells of Cajal (ICC) or their progenitor stem cells.(12) Immunohistochemistry has enabled their recognition as a unique group of gastrointestinal mesenchymal tumours by their cytoplasmic immunoreactivity for c-KIT protein (CD117).(12)
CD117 (KIT) protein is a transmembrane growth factor receptor belonging to the tyrosine kinase family whose ligand is the stem cell factor.(6,13) In the gastrointestinal tract immunohistochemical identification of the CD117 antigen is consistently demonstrable in the ICC (myofibroblasts). These cells are the autonomic nerve-related GI pacemaker cells dispersed between the intestinal smooth muscle cells that co-ordinate intestinal motility and peristalsis.(4,12,13)
Prior to their immunohistochemical characterization, GISTs were classified as leiomyomas, leiomyosarcomas, leioblastomas or schwannomas based on their histological appearance and apparent origin from the tunica muscular is.(13) It is now suggested that these ICC are the histogenic origin of the neoplastic mesenchymal cells in GISTs due to their immunohistochemical similarity producing diffuse strong cytoplasmic expression of CD117.(13,6) The variability in histologic appearance is likely due to different pathways of differentiation within these tumours.
GISTs are more frequently observed in the intestinal tract of the veterinary companion animals, in particular the cecum in horses in comparison to humans where the stomach is the most common site of origin.(4,6,7,13) In a prior study in dogs, GISTs occurred in older animals with no overt sex predisposition and a relatively low incidence of metastasis.(5)
In humans, GISTs tend to be more densely cellular than smooth muscle tumors and several histological patterns have been identified of which the most commonly described is the spindloid pattern composed of fusiform cells arranged in whorls or interlacing fascicles or whorls with elongated or cigar-shaped nuclei with perinuclear vacuoles.(14) Less commonly observed is the polygonal or epithelioid variant.(14)
These tumors can express CD34 and SMA in addition to the more consistent expression of CD117 and vimentin.(7) Desmin and S-100 are usually not expressed.(13) In contrast leiomyomas are typically positive for SMA and desmin which are markers of smooth muscle differentiation. These tumors do not express CD117 and usually have an indolent course.(6) Tumors that do not express SMA or CD117 have been described as GIST-like tumors.(11,12)
Prior reports of canine, equine and feline GISTs are histologically and immunohistochemically comparable with the human spindloid variant.(4,13) Smooth muscle differentiation resulting in the co-expression of SMA in some tumours supports the hypothesis that GISTs develop from a pluripotential ICC or its progenitor cell type which can differentiate into smooth muscle cells.(4,13)
Alteration of the gene encoding the tyrosine kinase receptor KIT is considered an early molecular event in the development of GISTs.(13) This enables ligand-independent receptor activation and subsequent signal transduction leading to cellular proliferation.(6) In human, canine and feline GISTs the majority of mutations have been identified in the juxtamembrane domain (exon 11) of the gene.(6,13) These KIT mutations are potential targets for therapeutic invention with tyrosine kinase inhibitors.(13)
It is difficult to determine the degree of malignancy of GISTs as their biological behaviour is not well characterised in animals.(13) In this case, following diagnosis, the cat was treated with toceranib phosphate (Palladia) for 10 months with no recurrence or metastatic spread of the tumor 2 years after surgical resection.
JPC Diagnosis:
1. Colon: Gastrointestinal stromal tumor.
2. Colon: Mast cell tumor, well-differentiated.
Conference Comment:
The contributor thoroughly discusses the immunohistochemical characteristics of GISTs, which became important in their differentiation from tumors of muscle origin. Although these are typically discovered as incidental findings at necropsy(4), this case portrays the magnitude to which these tumors can grow and cause functional obstructions as observed clinically in this cat. They have also been reported to induce paraneoplastic hypoglycemia by their production of insulin-like growth factors and erythrocytosis from their production of erythropoietin.(3)
An abdominal mass in the cat must also include the non-neoplastic lesion, eosinophilic sclerosing fibroplasia, as a possible differential which may also lead to obstructive clinical signs. This mass of eosinophils, fibroblasts and collagen may be found throughout the gastrointestinal tract in cats of all ages. It is hypothesized this is an inflammatory response to a bacteria and may be related to the cats affection for recruiting eosinophils in other dermatologic and oral conditions known collectively as the eosinophilic granuloma complex.(5)
References:
1. Atzwanger BL, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Arch 456:111-127, 2010.
2. Bettini G, Morini M. Marcato P. Gastrointestinal spindle cell tumors of the dog: Histological and immunohistochemical study. J Comp Path. 2003;129:283-293.
3. Brown C, Baker D, Barker I. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th edition. Vol. 2. 2007;127-128.
4. Cooper BJ, Valentine BA. Tumors of muscle. In: Meuten DJ, ed. Tumors in Domestic Animals. 4th ed. 2002;328-333.
5. Craig LE, Hardam EE, Hertzke DM, Flatland B, Rohrbach BW, Moore RR. Feline gastrointestinal eosinophilic sclerosing fibroplasias. Vet Pathol. 2009;46:63-70.
6. Frost D, Lasota J, Miettinen M. Gastrointestinal stromal tumors and leiomyomas in the dog: A histopathologic, immunohistochemical and molecular genetic study of 50 cases. Vet Pathol. 2003;40:42-54.
7. Gillespie V, Farrelly K, Craft D, Luong R. Canine gastrointestinal stromal tumors: Immunohistochemical expression of CD34 and examination of prognostic indicators including proliferation markers Ki67 and AgNOR. Vet Pathol. 2011;48:283-291.
8. Hanazono, K, Fukumoto S and Hirayama K. Predicting metastatic potential of gastrointestinal stromal tumors in dog by ultrasonography. J Vet Med Sci. 2012;74:1477-1482.
9. Head KW, Else RW, Dubielzig RR. Tumors of the alimentary tract. In: Meuten DJ, ed. Tumors in Domestic Animals. 4th edition. 2002;461-476.
10. Henry C, Herrera C. Mast cell tumors in cats. J Fel Med Surg. 2013;15:41-47.
11. Luc A, Prata D, Huet H, Lagadic M, Bernex F. A KIT positive gastrointestinal stromal tumor in a ferret (Mustela putorius furo). J Vet Diagn Invest. 2009;21:915-917.
12. Maas C, Haar G, Gaag I. Reclassification of small intestinal and caecal smooth muscle tumors in 72 Dogs: Clinical, histologic, and immunohistochemical evaluation. Veterinary Surgery. 2007;36:302-313.
13. Morini M, Gentilini F, Spadari A. Cytological, immunohistochemical and mutational analysis of a gastric gastrointestinal stromal tumor in a cat. J Comp Path. 2011;145:152-157.
14. Morini M, Bettini G, Preziosi R, Mandrioli L. C-kit gene product (CD117) immunoreactivity in canine and feline paraffin sections. Journal of Histochemistry & Cytochemistry. 2004;52:705708.