Signalment:
Gross Description:
Histopathologic Description:
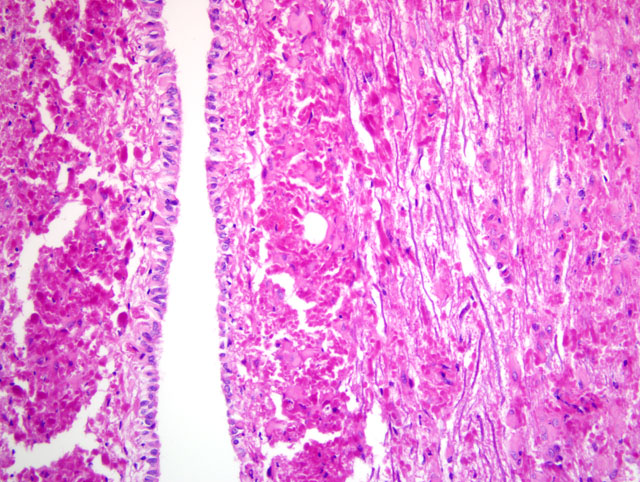
Within the cerebral cortex, RF were present in subpial locations. They were attended by low numbers of hypertrophied, and occasionally multinucleated astrocytes with stout fibrillar extensions into the pial membrane. Subcortical and central white matter did not contain RF, although there were a few hypertrophied astrocytes. However, RF density was high in the periventricular and subependymal regions around the third ventricle, and of lesser degree in the subependymal region of the temporal horns. The white matter of the hippocampus and corpus callosum contained occasional RF and mild astrocytic reaction. Perivascular RF were observed in the central gray matter.
The molecular layer of the cerebellum showed small numbers of subpial RF which, as in the cerebral gray matter, were attended by a few hypertrophied astrocytes. The granular layer, and more particularly the folial and deeper white matter, contained abundant RF and associated hypertrophied astrocytes.
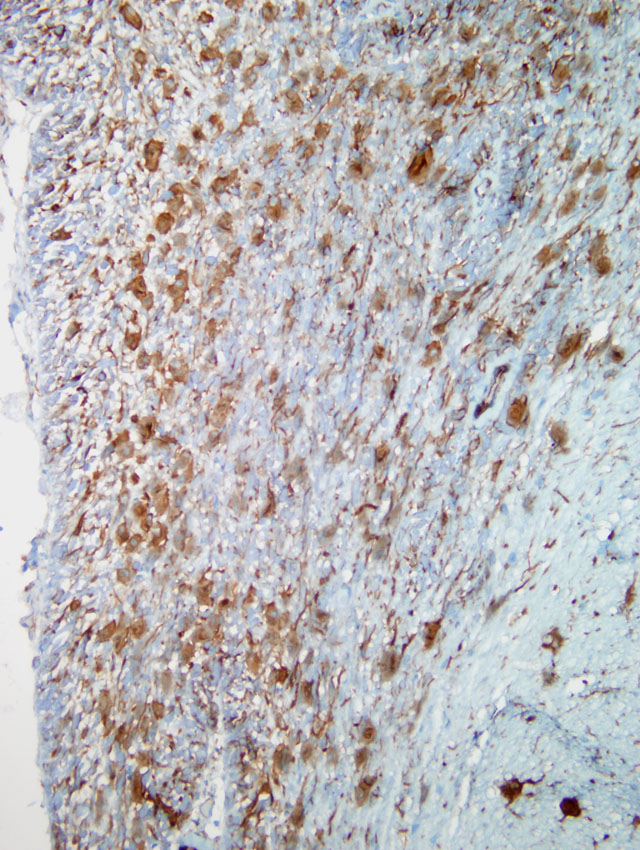
In the pons and medulla, there were prominent subpial, subependymal and perivascular RF aggregates, accompanied by numerous hypertrophied astrocytes, with lesser numbers in the white matter. Ependymal cells were hypertrophied. αB -crystallin immunostaining showed positive cytoplasmic expression in many glia, including a subset of astrocytes with a ring-like perinuclear pattern of staining. Many RF were immunonegative, except for a thin rim of positive staining. Many of the αB -crystallin-positive astrocytes were GFAP-negative.
Rosenthal fibers reacted strongly with ubiquitin, but were vimentin-negative. GFAP gradually replaces vimentin in astrocytic IF during brain development, although both may be coexpressed in reactive astrocytes and some astrocytic subpopulations. Rosenthal fiber HSP70 expression resembled that for αB-crystallin but normal astrocytic perikarya, unlike the latter, did not stain for HSP70.
Within the spinal cord, RF formation was florid, generally being greatest in perivascular and subependymal locations. However, in some areas, gray matter contained nearly as many RF as white matter. There were also moderate to large numbers of hypertrophied astrocytes, small numbers of axonal spheroids, and minimal Wallerian degeneration.
All neuronal populations in the brain and spinal cord, including those in cortical, central, brainstem and spinal gray matter, as well as the pyramidal and granule cells of the hippocampus and cerebellar Purkinje and granule cells, were of normal morphology. Oligodendroglial nuclei also appeared normal.
In sheep #2, the neuroanatomical distribution of RF was similar to sheep #1, but they were greater in number and density. There was mild, Wallerian degeneration in the spinal cord, with ellipsoids (digestion chambers) containing axon fragments, and sometimes macrophages. Myelin ellipsoids were found in all funiculi, but were sometimes more prominent in, or largely restricted to, the ventral funiculi.
Electron Microscopy Results: At the ultrastructural level, within astrocytes, RF were electron-dense, rounded or elongated, coarsely granular structures of variable size with irregular contours. They were surrounded by densely aggregated sheaths of IF, some of which appeared to be continuous with the central osmiophilic mass.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
The histopathological, immunohistochemical and ultrastructural features of the RF encephalomyelopathy described in these two sheep resembled those found in human AD cases and the AD-like encephalopathies previously reported in several dog breeds and one sheep. The age of the present sheep, neuroanatomical distribution of RF, and paucity of myelin loss was similar to adult-onset AD in humans and the only other AD-like neuropathy reported in sheep, while canine cases resembled the human juvenile form of AD. However, this is the first case of an AD-like encephalopathy in domestic animals to be subjected to molecular pathological analysis. In almost all humans with AD, a heterozygous mutation has been identified in exons 1 to 8 of the GFAP gene, and since the sheep GFAP gene has not been sequenced, fragments corresponding to the highly conserved regions of exons 1 to 9 of the bovine GFAP gene were amplified. Polymerase chain reaction results revealed numerous homozygous nucleotide variations in both the affected and control sheep when compared to the published bovine sequence, but these were considered to represent interspecies variations.
It is hoped that further cases of ovine AD from this property will become available for molecular and pathological evaluation so that the strongly suspected genetic basis for the disease may be confirmed and characterized.
JPC Diagnosis:
Conference Comment:
In humans, the infantile form of AD is characterized not only by RF accumulation, but by severe myelin changes in the frontal lobes, resulting in the characterization of AD as a leukodystrophy; this may be attributable to oligodendrocyte dysfunction secondary to the primary astrocytopathy. However, demyelination may or may not be present in the juvenile and adult forms, and pathology is primarily confined to the brainstem; thus, characterization as a leukodystrophy may not be appropriate in all cases.(3) As noted by the contributor, the lack of demyelination in the present case is reminiscent of the juvenile and adult forms of AD in humans.
References:
2. Brenner M, Johnson AB, Boespflug-Tanguy O: Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet 27:117-120, 2001
3. Cox NR, Kwapien RP, Sorjonen DC, Braund KG: Myeloencephalopathy resembling Alexanders disease in a Scottish terrier dog. Acta Neuropathol (Berl) 71:163-166, 1986
4. Fankhuaser R, Fatzer R, Bestetti G, Derauz JP, Perentes E: Encephalopathy with Rosenthal fiber formation in a sheep. Acta Neuropathol (Berl) 50:57-60, 1980
5. Summers BA, Cummings JF, De Lahunta A: Veterinary Neuropathology, pp. 281-283. Mosby, St. Louis, MO, 1995
6. Tomokane N, Iwaki T, Tateishi J, Iwaki QA, Glodman JE: Rosenthal fibres share epitopes with άB-crystallin, glial fibrillary acidic protein, and ubiquitin, but not with vimentin. Immunoelectron microscopy with colloidal gold. Am J Pathol 138:875-885, 1991
7. Weissenbock H, Obermaier G, Dahme E: Alexanders disease in a Bernese mountain dog. Acta Neuropathol (Berl) 91:200-204, 1996