Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 18
12 February, 2020
Timothy Cooper, DVM, DACVP
Pathology Department
NIH/NIAID
Integrated Research Facility
Frederick, MD
CASE III: WSC Case 1 (JPC 4049886).
Signalment: 22Y 6M 12D, female, common squirrel monkey (Saimiri sciureus).
History: Patient was found dead in its enclosure. Patient had been exhibiting abnormal behavior the day prior. A necropsy was performed and tissue specimens were submitted in formalin for histopathologic evaluation.
Gross Pathology: There were multiple, multifocal, up to 1 mm diameter dark red-black foci throughout the cerebral gray matter. A small amount of bruising and hemorrhage was noted in the rostral mandible.
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.): None.
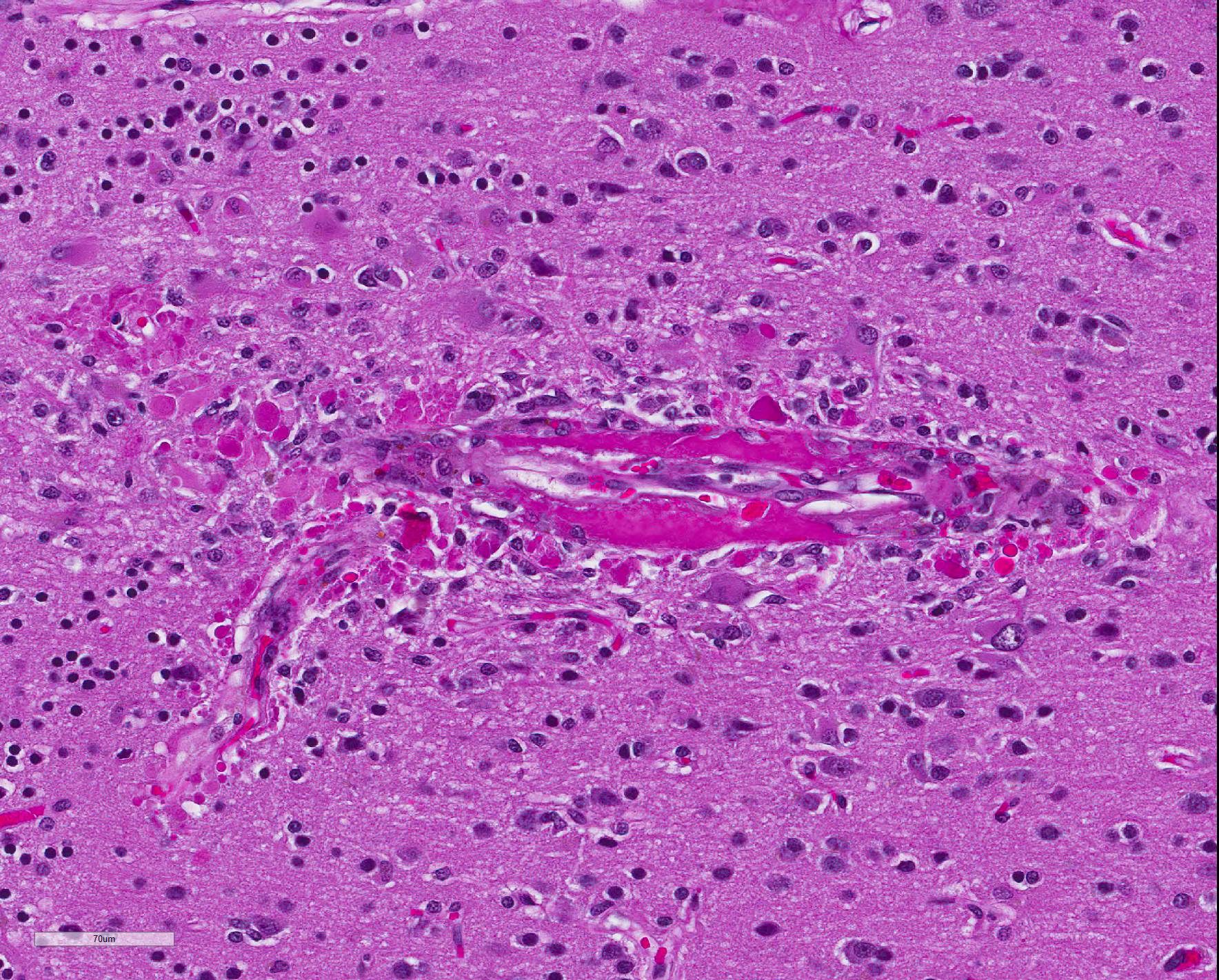
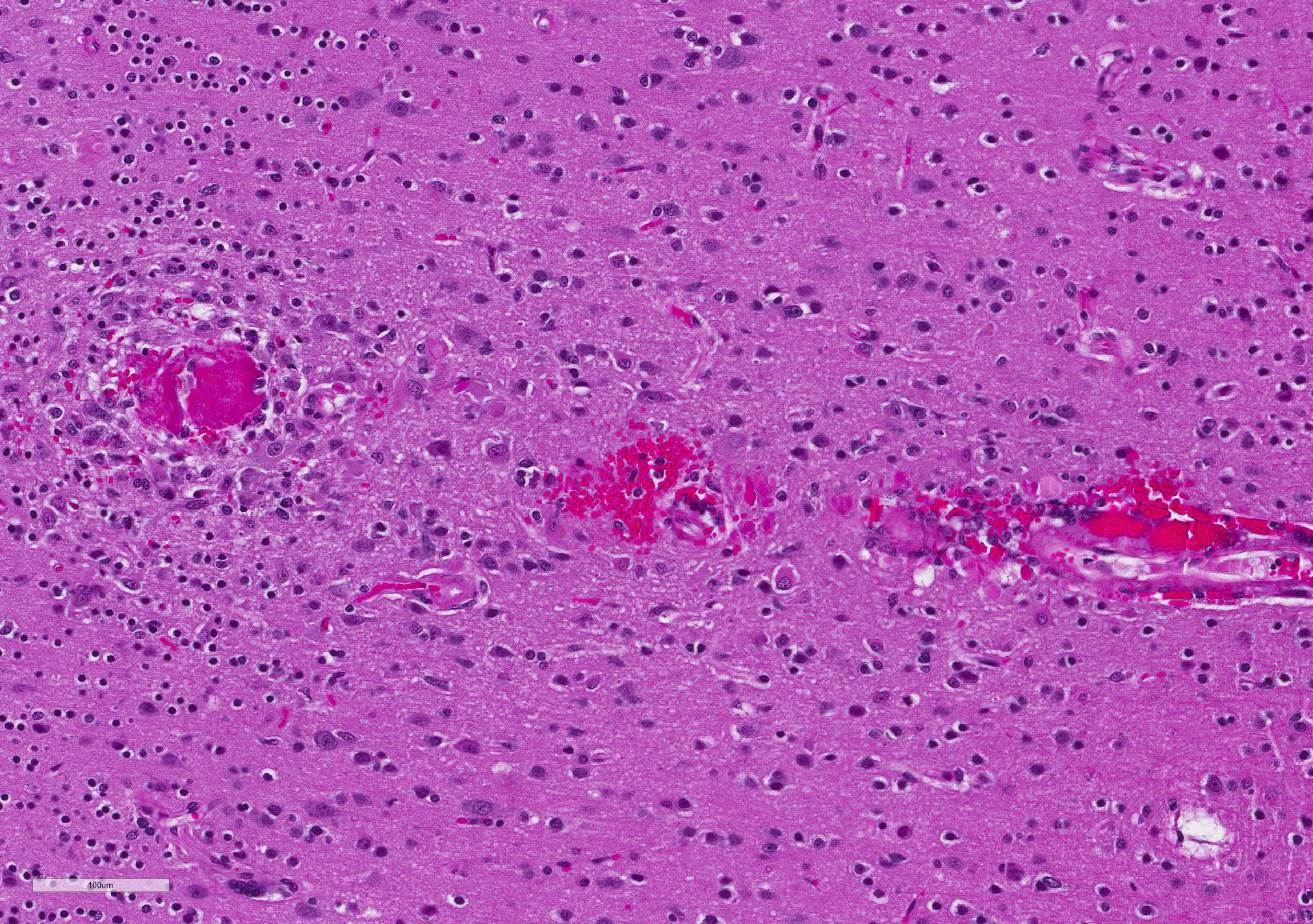
Microscopic Description: Diffusely, cerebral meningeal and parenchymal vessels are transmurally expanded and effaced by variable amounts of amorphous, finely fibrillar to waxy, pale eosinophilic material (amyloid). Multifocally, affected vessels are surrounded by mild to moderate amounts of fibrinoid exudate and acute to chronic hemorrhage as well as mild to moderate numbers of gitter cells that occasionally contain golden-brown globular intracytoplasmic pigment (hemosiderin), lymphocytes and plasma cells and fewer gemistocytic astrocytes and occasional multinucleate giant cells. There are multifocal small (10-25 um) round parenchymal deposits (senile plaques) often adjacent to affected vessels.
Contributor Morphologic Diagnosis:
Cerebrum, vessels: Amyloidosis, transmural, diffuse, severe, with hemorrhage, fibrinoid exudation, astrogliosis, senile plaques and occasional multinucleate giant cells.
Contributor Comment:
The histomorphologic findings in the cerebrum are consistent with cerebral amyloid angiopathy (CAA) ? a vascular disorder caused by the accumulation of amyloidogenic proteins in the walls of cerebral blood vessels.4 In severe cases, CAA can result in other vasculopathic changes such as microhemorrhages, fibrinoid extravasation and focal gliosis.4 In some cases, vascular/perivascular inflammation, fibrinoid necrosis and leukoencephalopathy have been associated with vascular β-amyloid deposition.4 The predilection to developing age-related CAA has been confirmed in the squirrel monkey and they are an animal model for CAA in humans in whom it has been shown to increase the risk of intracerebral bleeding and has been associated with up to 20% of non-traumatic hemorrhagic strokes in the elderly.4 Additionally, CAA is a risk factor for cognitive decline (even in the absence of stroke) and may worsen the dementia of Alzheimer?s disease (AD).4 Currently, there are no practical antemortem diagnostic tests or treatments for CAA. CAA-related vasculopathies arise in squirrel monkeys but are not common. In severely affected animals (as in this case), microhemorrhages and fibrinoid exudation may occur. Several studies support squirrel monkeys as an optimal biological model for idiopathic CAA and for testing the safety and efficacy of β-amyloid reduction strategies such as β-amyloid-immunization therapy for AD.4
The pathogenesis of CAA remains obscure; however, failure of active/passive elimination of β-amyloid from the aging brain may play an important role.4 Squirrel monkeys naturally develop β-amyloid deposits in the form of CAA and senile plaques starting at approximately 13 years of age.4 Their life span in captivity is up to 30 years. Additionally, their cerebrovascular and immune systems are similar to humans. The β-amyloid precursor protein in squirrel monkeys is 99.5% homologous to human βAPP751 and the amino acid sequence of β-amyloid is identical in the two species.2 Severely affected arterioles show loss of smooth muscle cells in the tunica media leading to weakening of the vascular wall and an increased propensity to rupture leading to intracerebral bleeding.2 Amyloid accumulation in cerebral vessels is known to induce degeneration of the entire neurovascular unit. Not only does the accumulation of insoluble amyloid species in vascular walls cause alterations of smooth muscle and endothelial cell layers, but amyloid deposition and concomitant microhemorrhages also occur in capillaries lacking the smooth muscle layer.5 Complement activation products co-localize with cerebral parenchymal and vascular deposits in AD and non- β-amyloid amyloidosis indicating that the chronic inflammatory response, most likely initiated by the deposits, is a general phenomenon.5 Once complement components are generated, they participate in several key steps of amyloidogenesis (aggregation, microglial activation and phagocytosis).5 Other markers of inflammation in AD include elevated cytokines and chemokines as well as accumulation of activated cytokine-expressing microglia found in or near pathologic lesions.5
Capillaries are especially vulnerable to CAA in squirrel monkeys.4 While in humans, leptomeningeal and cortical arteries and arterioles are especially vulnerable. Both (vessels and parenchyma) types of β-amyloid deposits occur primarily in the neocortex with the highest density of lesions in the rostral cortex diminishing caudally.4 Distribution and quantity of deposits are highly symmetrical in the right and left hemispheres.2 In the squirrel monkey aggregated β-amyloid occupies meningeal and parenchymal arteries/arterioles as well as numerous parenchymal capillaries.4 Few capillaries are congophilic and, as in humans, veins are seldom amyloidotic.4 In the arteriolar wall, the basal lamina of the tunica media is the primary site of β-amyloid accumulation.4 The tunica adventitia is also frequently involved.4 When severe, there is effacement of/hypocellularity of the vascular wall ? especially within the tunica media.4 In contrast to humans, large parenchymal senile plaques are relatively infrequent in aged squirrel monkeys and when they occur they are usually small (10-25 um in diameter) and spherical.4 Amyloidotic vessels occasionally may be surrounded by reactive microglia; however, they are more often associated with reactive astrocytes.4
CAA is ultrastructurally composed of classic amyloid fibrils and is the principal type of cerebral β-amyloidosis in squirrel monkeys.4 The basal lamina of amyloidotic vessels is enlarged and distended by masses of classic amyloid fibrils. In capillaries, β-amyloid accumulates in the basal lamina and tunica adventitia (due to lack of tunica media proper). In some cases, β-amyloid in parenchymal and vascular lesions is coextensive.4 Additional diagnostic tests include Congo Red special histochemical stain, β-amyloid immunohistochemistry, Thoflavin T fluorescent stain and the Shtrasburg method. CAA has also been reported in a sooty mangabey.5 This case was reviewed in consultation with Dr. Derek Mathis, MD neuropathologist.
Contributing Institution:
http://www.wpafb.af.mil/AFRL
JPC Diagnosis: Cerebrum, small and medium-caliber arteries: Amyloidosis , multifocal, moderate, with multifocal fibrinoid necrosis, and minimal perivascular granulomatous inflammation.
JPC
Comment: The contibutor has
done an excellent job in describing the syndrome of cerebrovascular amyloidosis
(cerebral amyloid angiopathy ? CAA) in the squirrel monkey, perhaps the best
animal model for a significant health problems in aging humans. Increasingly
sophisticated imaging technologies are available today for antemortem detection
CAA in squirrel monkeys. Magnetic resonance imaging has detected two distinct
patterns associated with cerebrovascular amyloid ? amyloid-related imaging
abnormalities (ARIA). T-2 weighted MRI has identified an edematous type
(ARIA-E), that appears as a hyperintense signal, and a hemosiderotic type
(ARIA-H) which demonstrates a hypointense signal. The ARIA-E type demonstrates
astrocytic and microglial hypertrophy in the white and grey matter.6
In humans, β-amyloid
peptide (Aβ) is an integral part (along with tauopathy) in the development
of Alzheimer?s disease, and the misfolding of Aβ is considered the
initiating event in the development of Alzheimer?s disease (AD).6
On its own, it is not associated with dementia or neurodegeneration in humans6,
but is considered a major cause of spontaneous intracerebral hemorrhage.1
CAA is commonly seen in elderly humans, and advancing age is the strongest risk
factor for its development.1 (Another risk factor, shared with AD,
is the apoliporotein E (ApoE) e4 allele).4 Up to 40% of
non-demented and 60% of demented individuals between the ages of 80 and 90
years may demonstrate CAA at autopsy.1 One study suggests that it
may be seen in up to 95% of humans with dementia if looked for diligently. A
number of manifestations of CAA in humans in the absence of Alzheimer?s disease
have been described, to include spontaneous cortical hemorrhage (with
associated microbleeds and siderosis), post-hemorrhage cognitive impairment,
and transient focal neurological episodes (?amyloid spells?).1 A
recent study demonstrated significant cortical thinning in individuals with
CAA.3
As mentioned above in context with the squirrel monkey, the currently accepted pathogenesis for CAA is not overproduction of Aβ, but deficiencies in clearance. Excess interstitial fluid in the brain is drained along arteries in the brain, bolstered by the pulsation of these vessels. The predominant theory of deposition is that as this perivascular drainage system fails with age, Aβ is progressive trapped in the expanding perivascular compartment allows for aggregation and deposition along vascular basement membranes.1
Regarding the comparative pathology of CAA, in addition to the squirrel monkey, there are a number of mouse models which overexpress Aβ and develop senile plaues and CAA, but no significant neurodegeneration.7 A number of researchers have suggested species-specific differences in post-translational characteristics and the the formation of species specific Aβ isoforms as a potential reason for the lack of associated neurodegenerative disease in some species. Squirrel monkeys also manifest extensive Aβ deposition, but not the additional lesions associated with Alzheimer?s disease.7
The
concurrent presence of amyloidosis and fibrinoid necrosis was interesting in
this case. The moderator described histologic differences in the two which are
best appreciated without the use of a condensor. In addition, the moderator
demonstrated the presence of giant cells adjacent to arteries containing mural
amyloid, as well as the discontinuity of arterial walls when stained with
smooth muscle actin (most appreciable on arteries cut on longitudinal section.
References:
1. Charidimou A, Boulouis G, Gurol ME, Ayata C, Bacskai, Frosch MP, Viswanathan A, Greenberg. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain 2017; 140:1829-1850.
2. D?Angelo OM, Dooyema J, Courtney C, Walker LC and Heuer E. Cerebral amyloid angiopathy in an aged sooty mangabey (Cercocebus atys). Comp Med (2013) 63(6): 515-520.
3. DeSimone CV, Graff-Radford J, El-Harasis MA, Rabinstein AA, Asirvatham SJ, Homes DR. Cerebral amyloid angiopathy: diagnosis, clinical implications, and management strategies in atrial fibrillation. J Am College Cardiol 2017; 70(6):1173-1181.
4. Elfenbein HA, Rosen RF, Stephens SL, Switzer RC, Smith Y, Pare J, Mehta PD, Warzok R and Walker LC. Cerebral β-amyloid angiopathy in aged squirrel monkeys. Histol Histopathol (2007) 22:155-167.
5. Ghiso J, Fossati S and Rostagno A. Amyloidosis Associated with Cerebral Amyloid Angiopathy: Cell Signaling Pathways Elicited in Cerebral Endothelial Cells. J Alzheimers Dis (2014).
6. Heuer E, , Jacobs J, Du R, Wang S, Kiefer OP, Cintron AF, Dooyemy J, Meng Y, Zhang X, Walker L. Amyloid related imaging abnormalities (ARIA) in an aged squirrel monkey with cerebral amyloid angiopathy. J Alzheimers Dis 2017; 57(2): 519-530.
7. Rosen RF, Tomidokoro Y, Farberg AS, Dooyema, Ciliax B, Preuss Tm, Neubert, TA, Ghiso JA, LeVine H, Walker LC. Comparative pathobiology of Aβ and the unique susceptibility of humans to Alzheimer?s disease. Neurobiol Aging 2016; 44:185-196.