CONFERENCE 24, CASE 1
Signalment:
120-day-old, male, commercial swine (Sus scrofa domesticus)
History:
Two growing-finishing farms in the Midwest region of Brazil experienced outbreaks of sudden death among pigs aged between 120 and 130 days. The mortality rate ranged between 9% and 10% in the affected batches. Upon clinical examination, some pigs exhibited nonspecific signs such as trembling, dyspnea, and squealing sounds shortly before death. Pigs from multiple pens of both sexes, especially the largest animals in the batch,
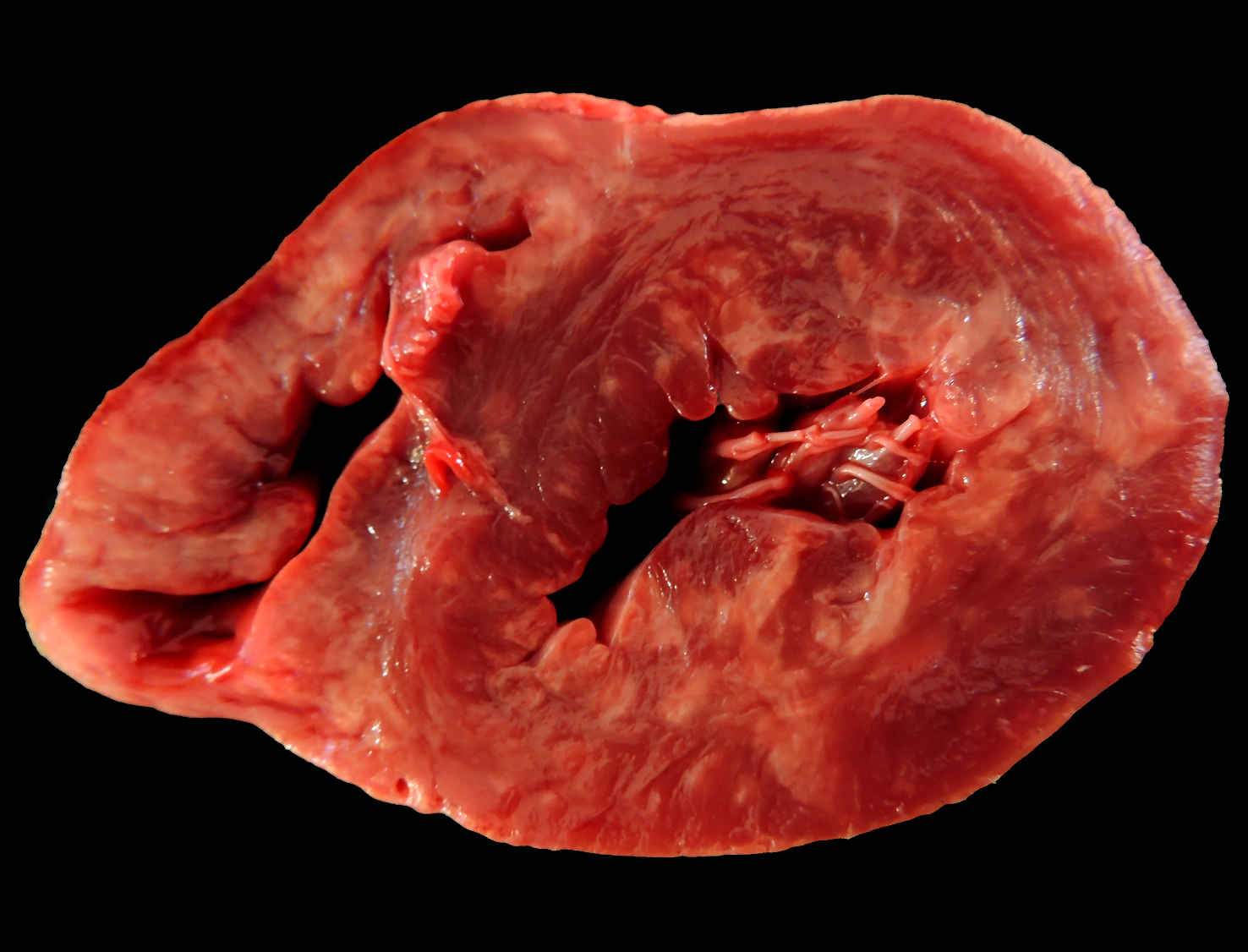
Heart, pig: On the cut surface of the myocardium, there were areas of pale tan discoloration ranging from 0.5-1 cm in diameter, sometimes coalescing, and more pronounced in the right and left ventricles. (Photo courtesy of Faculdade de Veterinária, Universidade Federal do Rio Grande do Sul, Setor de Patologia Veterinária, http://www.ufrgs.br/patologia).
were affected. The antimicrobial and antipyretic treatment used did not yield any response. Additionally, large numbers of black rats (Rattus rattus) were observed inhabiting the facilities during farm visits. The rodent control measures implemented on the farm were ineffective.
Gross Pathology:
At necropsy, major lesions were observed in the heart, which was enlarged and showed on the epicardial surface multifocal pale to slightly white areas, ranging from 0.5-1 cm in diameter, which extended through the myocardium on the cut surface. These pale areas were more pronounced in the right and left ventricles, occasionally presenting a gritty texture. Mild hydropericardium and pulmonary edema were also observed.
Laboratory Results:
RT-PCR: samples of liver, lymph node, and heart tested positive for Encephalomyocarditis virus. Brain sample tested negative.
Microscopic Description:
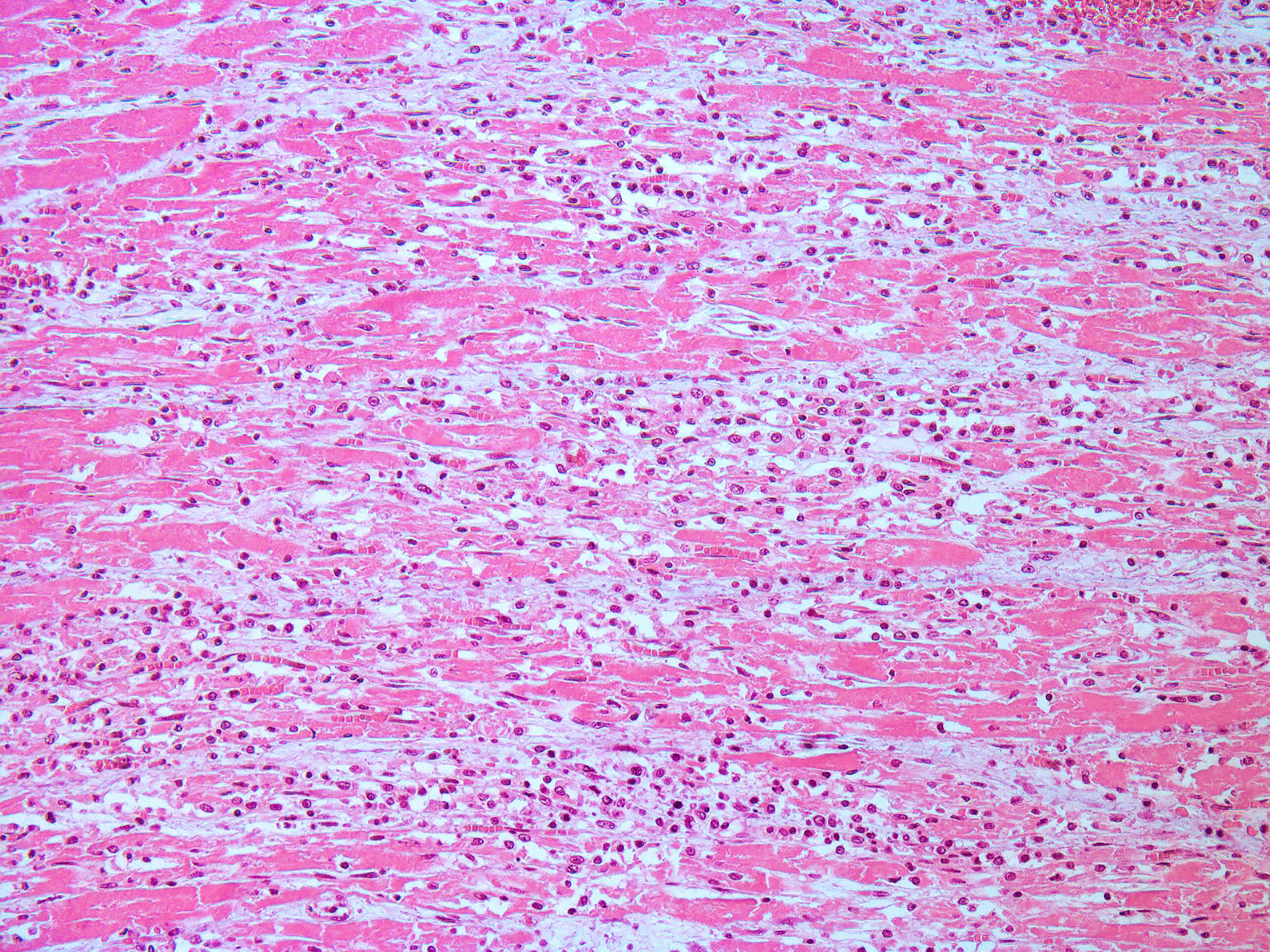
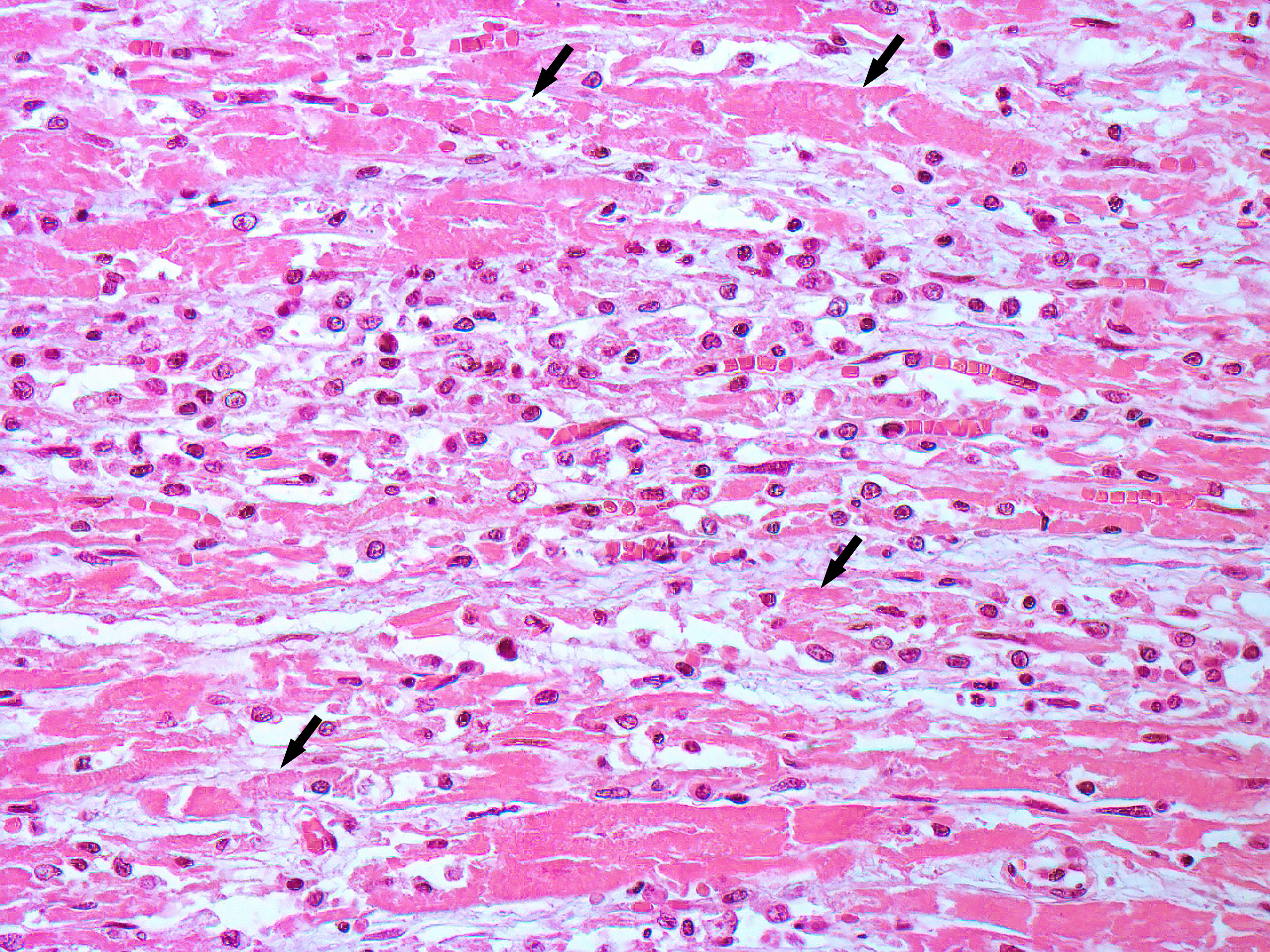
Heart, left ventricle: in the myocardium, multifocally, there are areas of necrosis of the cardiomyocytes, characterized by hypereosinophilia, loss of cellular detail, nuclear karyolysis and pyknosis, associated with non-suppurative inflammation, composed of macrophages, lymphocytes and plasma cells. This inflammatory infiltrate frequently replaces the necrotic cardiomyocytes and infiltrates the myocardial interstitium. In some areas, there is deposition of intracytoplasmic basophilic granular material in necrotic cardiomyocytes (highlighted by Von Kossa histochemistry - mineralization). Additionally, mild fibrous connective tissue proliferation is also present in the myocardial interstitium.
Contributor’s Morphologic Diagnosis:
Myocarditis, necrotizing and lymphoplasmohistiocytic, multifocal, moderate, with dystrophic mineralization.
Contributor’s Comment: The Picornaviridae family consists of small, non-enveloped, single-stranded RNA viruses, which include the Cardiovirus genus. Encephalomyocarditis virus (EMCV) is classified within the genus Cardiovirus, specifically as Cardiovirus A (the genus Cardiovirus comprises six species named A to F).5,9
EMCV is cause of myocarditis in several wildlife species, including non-human primates, as well as domestic animals, particularly swine, and humans.1,3,4,6,11 Although the name 'encephalomyocarditis' originates from the initial association of the virus with brain and cardiac lesions, encephalitis is not consistently observed in natural EMCV infections in pigs.8,12 Encephalitis mainly occurs in experimental infections in mice and swine fetuses, whereas myocarditis is a frequent lesion in both natural and experimental infections.1,8,12
Rats and mice serve as reservoir species for EMCV, spreading the virus and causing outbreaks in susceptible animals.3,7,8 Several dead black rats (Rattus rattus) from the reported farms were subjected to necropsy, revealing positive RT-PCR results for EMCV in fecal samples. Accordingly, the presence of rodent infestation at the farms where the outbreak occurred indicates a failure of the control system and suggests rodents as potential reservoir and disseminator of EMCV in this report. On the other hand, EMCV infection is usually subclinical and may demonstrate that recovered pigs play an important role in the dissemination of EMCV. Therefore, in addition to controlling rodents, it is crucial to identify subclinical animals for more effective disease control.2
EMCV infection can result in characteristic myocardial lesions, with a pattern of viral myocarditis (non-suppurative inflammation), myocardial necrosis, and marked dystrophic mineralization which are clues for histopathological diagnosis.8,12 However, some differential diagnoses should be considerate such as Aphthovirus infection, Parvovirus infection, and vitamin E/selenium (VitE/Sel) deficiency.12,14 VitE/Sel deficiency is the most important differential diagnosis for commercial pigs and can also lead to cardiac mineralization patterns similar to those seen in EMCV infection. However, VitE/Sel deficiency is more common in nursery pigs compared to finishing pigs, and other lesions such as cardiac hemorrhages, hepatic necrosis, and muscular necrosis may be present in this condition, which are useful for pathological differentiation.10,12 Additionally, RT-PCR detection of EMCV is crucial to confirm the diagnosis.8
In Brazil, the first occurrence of EMCV in pigs was reported in 1985 on a farrowing farm in the southern region, affecting one sow and resulting in the death of 9 out of 10 piglets.13 After a 38-year gap with no further documented cases, an outbreak in growing-finishing pigs occurred in 2023.8 The phylogenetic analysis of the VP1 gene from the EMCV identified in this outbreak, showed that the samples contained similar strains that had their closest relatives identified in humans in Peru.6,8 However, epidemiological, serological, and field studies on the distribution of EMCV in Brazil are limited, and more research is needed to understand the real impact of EMCV.
Contributing Institution:
Faculdade de Veterinária
Universidade Federal do Rio Grande do Sul
Setor de Patologia Veterinária
http://www.ufrgs.br/patologia
JPC Morphologic Diagnosis: Heart, ventricle: Pancarditis, necrotizing and lymphohistiocytic, monophasic, subacute, multifocal, marked.
JPC Comment: This week’s moderator was Dr. Patty Pesavento from the University of California-Davis, who explored viral and vascular pathology with conference participants. We enjoyed discussing this first case as its descriptive features reinforced the underlying pathogenesis of EMCV. Dr. Pesavento’s first question to the group challenged them to consider the target cell for the virus – is it the myocyte or something else?
The answer in this case is probably both. Prior research has shown that vascular cell adhesion molecule 1 (VCAM1) is the necessary receptor for EMCV infection,16 though other sialylated surface glycoproteins may play a role depending on EMCV strain. Supporting this interpretation of endothelial cells within capillaries being a target is the distribution of changes across all layers of the heart (i.e. pancardial) and regionally distinctive areas of necrosis and mineralization that are suggestive of damage to small-caliber blood vessels. A Movat’s pentachrome stain highlights that much of the increased clear space in section reflects edema versus fibrosis.
We appreciate the contributors’ morphologic diagnosis and added a single modifier to qualify the necrosis as monophasic in this case. The dystrophic mineralization in this case is remarkable and we speculate that the punctate basophilic foci may represent mineralization of organelles (mitochondria) secondary to ATP depletion and calcium dysregulation.
The contributor provides several good differential diagnoses for this case. Other important rule outs for porcine myocarditis (pancarditis) include porcine circovirus 2, porcine parvovirus, and toxin ingestion such as cottonseed (gossypol).12 Dr. Pesavento also noted two other important scenarios related to transmission of EMCV for participants to be aware of. As rodents are a common nuisance in zoological parks, they may contaminate feed and water with virus, or in select cases, be ingested directly by primates or other species.3 Finally, EMCV has been shown to persist within the porcine myocardium, which presents an unusual hazard for xenotransplantation of hearts to cardiac patients.15 As recipients are highly immunosuppressed, the potential for subsequent permissive replication and spread to the CNS leading to severe encephalitis should not be overlooked.
References:
- Alexandersen S, Knowles NJ, Belsham GJ, et al. Picornaviruses. In: Diseases of Swine. John Wiley & Sons, Ltd 2019:641–684.
- Billinis C, Paschaleri-Papadopoulou E, Psychas V, et al. Persistence of encephalomyocarditis virus (EMCV) infection in piglets. Vet Microbiol. 1999;70(3-4):171-177.
- Canelli E, Luppi A, Lavazza A, et al. Encephalomyocarditis virus infection in an Italian zoo. Virol J. 2010;7(1):64.
- Cardeti G, Mariano V, Eleni C, et al. Encephalomyocarditis virus infection in Macaca sylvanus and Hystrix cristata from an Italian rescue centre for wild and exotic animals. Virol J. 2016;13(1):193.
- Carocci M, Bakkali-Kassimi L. The encephalomyocarditis virus. Virulence. 2012;3(4):351–367.
- Czechowicz J, Huaman JL, Forshey BM, et al., Prevalence and risk factors for encephalomyocarditis virus infection in Peru. Vector Borne Zoonotic Dis. 2011;11(4):367–374.
- Foglia EA, Pezzoni G, Bonilauri P, Torri D, Grazioli S, Brocchi E. A recent view about encephalomyocarditis virus circulating in compartmentalised animal population in Northern Italy. Sci Rep. 2023;13(1):592.
- Gris AH, Alves RS, Camargo LJ, et al. Reemerging of Encephalomyocarditis Virus in Pigs in Brazil: Pathological and Viral Characterization. Transbound Emerg Dis. 2023;2023:1–6.
- Lefkowitz EJ, Dempsey DM, Hendrickson RC, Orton RJ, Siddell SG, Smith DB. Virus taxonomy: the database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018;46(D1):D708–D717.
- Menegatt JCO, Perosa FF, Gris AH, et al. Main Causes of Death in Piglets from Different Brazilian Nursery Farms Based on Clinical, Microbiological, and Pathological Aspects. Animals. 2023;13(24):3819.
- Oberste MS, Gotuzzo E, Blair P, et al. Human Febrile Illness Caused by Encephalomyocarditis Virus Infection, Peru. Emerg Infect Dis. 2009;15(4):640–646.
- Robinson WF, Robinson NA. Chapter 1 - Cardiovascular System. In: Maxie MG, ed. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals: Volume 3 (Sixth Edition). W.B. Saunders 2016:1-101.e1.
- Roehe PM, Rodrigues NC, Oliveira SJ de, et al. Encephalomyocarditis virus (EMCV) in swine in the state of Rio Grande do Sul, Brazil. Rev Argent Microbiol. 1985;16(2):117–120.
- Scollo A, Mazzoni C, Luppi A. Management of encephalomyocarditis virus infection in Italian pig farms: a case report. BMC Vet Res. 2023;19(1):54.
- Brewer LA, Lwamba HC, Murtaugh MP, Palmenberg AC, Brown C, Njenga MK. Porcine encephalomyocarditis virus persists in pig myocardium and infects human myocardial cells. J Virol. 2001 Dec;75(23):11621-9.
- Huber SA. VCAM-1 is a receptor for encephalomyocarditis virus on murine vascular endothelial cells. J Virol. 1994 Jun;68(6):3453-8.