Signalment:
Histopathologic Description:
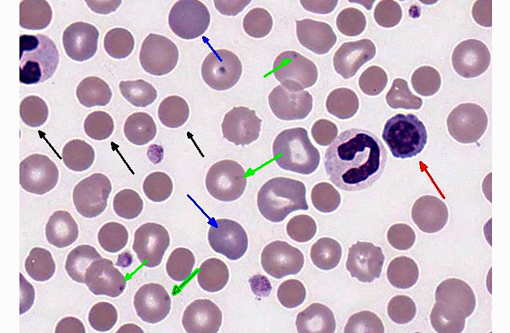
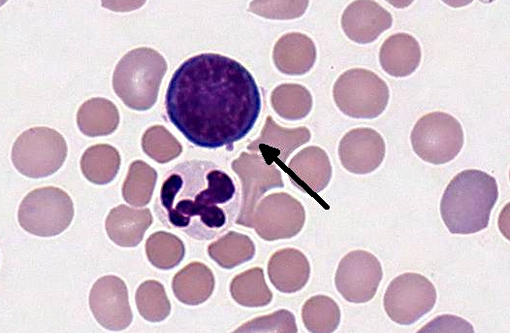
Leukon: There is a moderate to marked leukocytosis, characterized by a neutrophilia with a left shift (including bands and metamyelocytes) and a monocytosis. There is evidence for toxic change in the neutrophils, particularly the early stages, based on the presence of D+â-¦hle bodies and intracytoplasmic granules.
Thrombon: The platelet mass appears adequate.
Morphologic Diagnosis:
1. Strongly regenerative anemia with evidence for probable immune-mediated destruction.
2. Inflammatory leukogram (leukemoid response).
3. Adequate platelet mass.
Overall Interpretation: Immune mediated hemolytic anemia (IMHA) with secondary leukemoid response.
Lab Results:
| Parameter | Value | Reference Interval |
| HCT | 21.9 % ↓ | 35.0-57.0 % |
| RBC | 3.1 x 106/μL ↓ | 4.95-7.87 x 106/μL |
| HgB | 8.2 g/dL ↓ | 11.9-18.9 g/dL |
| MCV | 79.4 fl | 69-80 fl |
| MCHC | 31.5 g/dL ↓ | 32.0-36.3 g/dL |
| WBC | 62.1 x 103/μL ↑ | 5.5-13.9 x 103/μL |
| Segs | 46.6 x 103/μL ↑ | 2.9-12.0 x 103/μL |
| Bands | 9.9 x 103/μL↑ | 0.0-0.45 x 103/μL |
| Lymphocytes | 1.2 x 103/μL | 0.4-2.9 x 103/μL |
| Monocytess | 4.3 x 103/μL ↑ | 0.1-1.4 x 103/μL |
| Eosinophils | 0 x 103/μL | 0.0-1.3 x 103/μL |
| nRBC | 17 (per 100 WBC) | |
| Reticulocytes | 9.4 % | |
| Absolute retics | 291 x 103/μL | |
| PLT | 185 x 103/μL | 235-694 x 103/μL |
Condition:
Contributor Comment:
Numerous breed predispositions have been discovered for development of primary IMHA, including Cocker Spaniels, Old English Sheepdogs, Border Collies, Poodles, Irish Setters, and Miniature Schnauzers. One study suggests an association between disease development and the genetic structure of the major histocompatibility complex.(4) The average age of onset is 6-8 years and, although there is no clear gender predisposition, it can be precipitated by the stress of heat or whelping.(2)
Affected animals can present with either chronic disease with nonspecific signs that have been present for days to weeks or they can present with acute illness, with severe signs of illness that have developed in one to two days. The chronic form is most commonly associated with extravascular hemolysis, while animals with acute disease typically exhibit signs secondary to intravascular hemolysis (e.g., icterus, hemoglobinemia, and hemoglobinuria).(2)
Diagnosis of IMHA begins with the examination of an EDTA anticoagulated blood sample for autoagglutination. This can be done both grossly and with a saline dilution. Microscopic examination of the blood smear typically shows a decreased red cell mass, usually with marked regeneration. The presence of abundant spherocytes is strongly suggestive for IMHA, although not pathognomonic, as these can be seen with other conditions, including zinc toxicity, DIC, and hemangiosarcoma.(3) A pronounced neutrophilia with a left shift is often seen in affected animals and is thought to be due to the effect of pro-inflammatory cytokines (e.g., IL-1, IL-6, and TNF-α), which are produced by activated macrophages. Tissue necrosis due to secondary thromboembolic disease may also play a role.(8) A Coombs test using species specific serum that recognizes patient IgM, IgG, and complement C3 can be of value in further characterizing the underlying mechanism.
Recent studies have evaluated the outcome of dogs with IMHA.(5,7,10) Around 50% of affected animals die during initial hospitalization, with much worse outcome for patients with the acute versus the chronic form of the disease. The presence of gross autoagglutination, a profound neutrophilia with a left shift, and concurrent thrombocytopenia have been associated with worse prognosis. The average survival time of those patients surviving the initial insult is just over one year. However, approximately 25% of patients have good long-term survival with appropriate immunosuppressive therapy.(10)
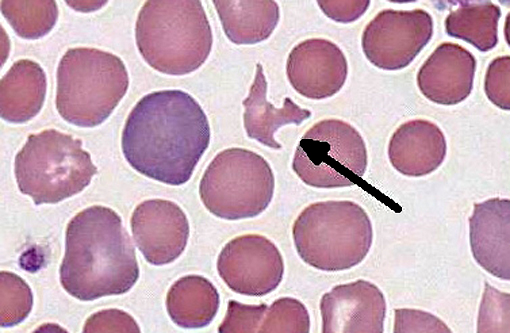
The patient in this case presented with the chronic form of the disease. While there was no gross or microscopic evidence for autoagglutination, examination of her peripheral blood revealed many of the classic features for immune-mediated disease, including abundant spherocytes and a strongly inflammatory leukogram. The presence of schistocytes and the absence of evidence for intravascular hemolysis is suggestive for antibodies to Fc receptors that are removed by splenic and hepatic macrophages, as schistocytes are often seen circulating secondary to membrane phagocytosis by activated macrophages.(6) To date, this patient has responded well to immunosuppressive therapy.
JPC Diagnosis:
1. Peripheral blood smear, erythron: Severe regenerative anemia with spherocytosis, schistocytosis and metarubricytosis, consistent with hemolysis.
2. Peripheral blood smear, leukon: Inflammatory leukogram with significant left shift and toxic neutrophil change.
Conference Comment:
Interestingly, avian species mount the most intense regenerative erythrocyte response, followed (in decreasing order) by dogs, cats, cows and horses. The presence of nucleated erythrocytes on a stained blood smear is classified as an appropriate response in animals with a strongly regenerative anemia or increased erythropoiesis; in these cases nucleated red blood cells are accompanied by reticulocytosis. Additionally, moderate numbers of nucleated red blood cells are normal in the peripheral blood of healthy piglets, and of course all erythrocytes in birds and reptiles are nucleated. On the other hand, metarubricytosis associated with iron, lead and copper toxicosis; hemangiosarcoma; leukemia; bone marrow disease; intervertebral disc disease; hereditary macrocytosis of poodles; endotoxemia; and FeLV is considered an inappropriate response.(1)
The most common causes of regenerative anemia are hemolysis and blood loss. Reticulocytosis is generally more severe with hemolysis, because iron from hemolyzed erythrocytes is more readily available for erythropoeisis than storage forms of iron, which must be mobilized when there is external loss of erythrocytes. Hemolysis is further classified as extra- or intravascular. Extravascular hemolysis, which is much more common, results from phagocytosis or lysis of erythrocytes within the spleen or liver, whereas intravascular hemolysis is erythrocyte destruction within the circulation. Schistocytes, or fragmented red blood cells, often indicate a microangiopathic hemolytic anemia (MAHA) and intravascular hemolysis1; however, as noted by the contributor, they may result from membrane phagocytosis, as is likely in this case. Serum chemistry and urinalysis results (not provided in this case) can also be helpful in distinguishing extravascular from intravascular hemolysis; hyperbilirubinemia is typically associated with extravascular hemolysis, whereas hemoglobinemia and bilirubinuria occur subsequent to intravascular hemolysis.
As noted by the contributor, the severe neutrophilia with a left shift and toxic change in neutrophils present in this case is common in IMHA. Four manifestations of toxic change in canine neutrophils are cytoplasmic basophilia, vacuolation, D+â-¦hle bodies and toxic granulation.(9)
In conclusion, this case illustrates several distinctive clinicopathological findings that permit the diagnosis of IMHA, and underscores the importance of performing a manual differential count on all blood smears. Some conference participants detected a lymphopenia based upon their manual differential counts, despite a count within the reference interval on the automated CBC. Participants speculated on possible explanations for this discrepancy, including the possibility that nucleated erythrocytes were erroneously categorized as lymphocytes on the automated counter.
References:
2. Day MJ. Immune-mediated anemias in the dog. In: Weiss DJ, Wardrop KJ, eds. Schalms Veterinary Hematology. 6th ed. Ames, IA: Blackwell Publishing; 2010:216-225.
3. Harvey JW. Erythrocytes. In: Atlas of Veterinary Hematology. Philadephia, PA: Saunders; 2001:31-32.
4. Kennedy LJ, Barnes A, Ollier WER, Day MJ. Association of a common dog leucocyte antigen class II haplotype with canine primary immune-mediated haemolytic anaemia. Tissue Antigens. 2006;68:502-508.
5. Piek CJ, Junius G, Dekker A, Schrauwen E, Slappendel RJ, Teske E. Idiopathic immune-mediated hemolytic anemia. Treatment outcome and prognostic factors in 149 dogs. J Vet Intern Med. 2008;22:366-373.
6. Rebar AH, Lewis HB, DeNicola NB, Halliwell WH, Boon GD. Red cell fragmentation in the dog: an editorial review. Vet Pathol. 1981;18:415-426.
7. Reimer ME, Troy GC, Warnick LD. Immune mediated hemolytic anemia: 70 cases (1988-1996). J Am Anim Hosp Assoc. 1999;35:384-391.
8. Scott-Moncrieff, Treadwell NG, McCullough SM, Brooks MB. Hemostatic abnormalities in dogs with primary immune-mediated hemolytic anemia. J Am Anim Hosp Assoc. 2001;37: 220-227.
9. Webb JL, Latimer KS. Leukocytes. In: Latimer KS, ed. Duncan and Prasses Veterinary Laboratory Clinical Pathology. 5th ed. Ames, IA: Wiley Blackwell; 2011:45-80.
10. Weinkle TK, Center SA, Randolph JF, Warner KL, Barr SC, Erb HN. Evaluation of prognostic factors, survival rates, and treatment protocols for immune-mediated hemolytic anemias in dogs: 151 cases (1993-2002). J Am Vet Med Assoc. 2005;226:1869-1880.