Signalment:
After weeks of supportive care and physical therapy, the dog was able to eat on his own. He continued to improve at home, though he lacked a menace response in the left eye and intermittently circled to the left. After five months, his neurologic status rapidly deteriorated, with incessant circling, hypersalivation, and multiple seizures. The owners elected euthanasia and histopathologic evaluation of the brain.
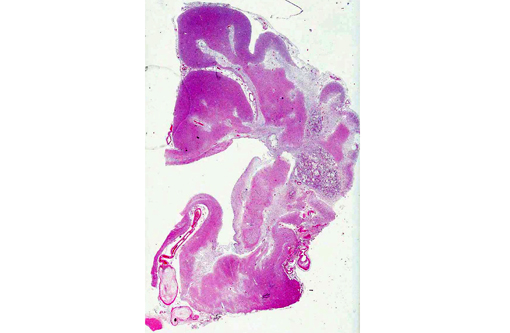
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
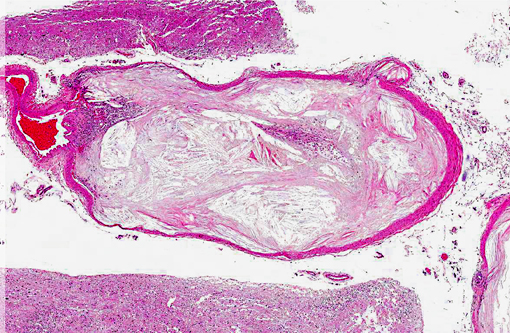
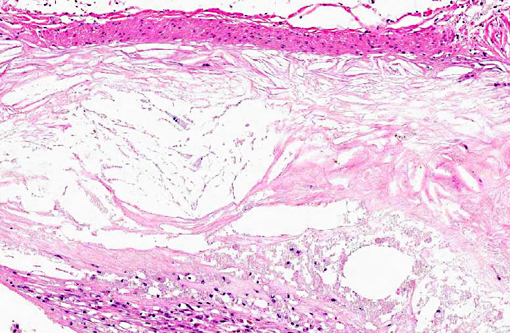
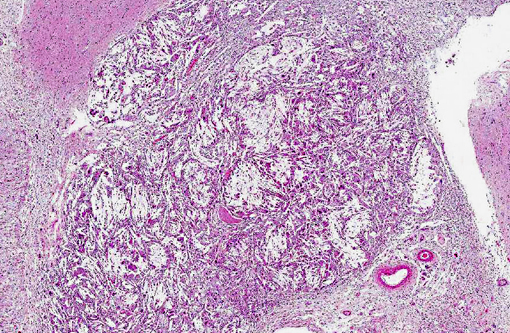
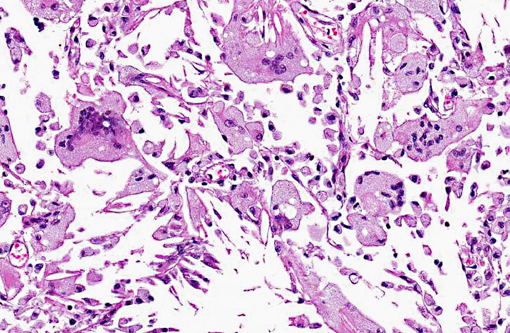
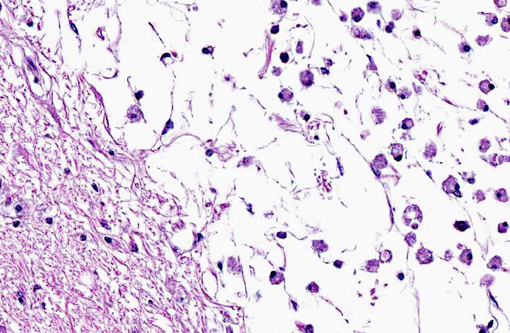
Leptomeningeal blood vessels: severe multifocal chronic atherosclerosis.
Condition:
Contributor Comment:
Lesions in dogs have been described within the aorta and within small muscular arteries in a variety of organs, including the heart, lung, alimentary tract, spleen, eye, and urogenital and endocrine organs.(6,8) Arteries in the heart, brain, and kidneys are usually most severely affected. Clinical disease is rare and occurs when, as in humans, the atheromas occlude or rupture into the lumen, leading to ischemia, thrombosis, and/or lipid embolism.(10)
On gross examination, affected arteries are yellow-white with thick, irregularly nodular walls and narrowed lumina. Microscopically, early lesions consist of lipid-laden macrophages and leiomyocytes (foam cells) admixed with degenerating leiomyocytes within the tunica media.8 With progression, the media becomes thicker with replacement of the normal architecture by intra- and extracellular lipid, cholesterol clefts, mineral, cellular debris, and fibrous connective tissue that may become hyalinized.(6,8,10) Severe lesions lead to extension into the adventitia, as well as disruption of the internal elastic lamina and extension into the intima. This is in contrast to human atherosclerosis, where lipid deposition occurs primarily in the intima.(10)
In almost all canine cases, atherosclerosis occurs in association with hypothyroidism and/or diabetes mellitus.10 Hypercholesterolemia is a common finding in both of these endocrinopathies and is thought to lead to the development of atherosclerosis in affected dogs.(5,10) However, in some dogs, serum cholesterol is normal and no endocrinopathy can be identified.(5) Furthermore, some thyroidectomized dogs on a high-cholesterol diet do not develop atherosclerosis.(10) Thus, other factors, such as genetics, may play a role in the development of canine atherosclerosis. In humans, a genetic predisposition to hyperlipidemia has been shown to increase the risk of atherosclerosis. While certain dog breeds, such as miniature schnauzers and Shetland sheepdogs, have a genetic predisposition to hyperlipidemia, additional studies are needed to determine the risk of atherosclerosis in these dogs.(5)
JPC Diagnosis:
Brain, cerebral arteries: Atherosclerosis, diffuse, severe.
Conference Comment:
There are five major plasma lipids in domestic animals: cholesterol, cholesterol esters, triglycerides and phospholipids are transported as lipoproteins, while nonesterified fatty acids are bound to albumin for transportation.(4) In mammals, dietary lipid is digested by pancreatic lipase and emulsified by bile acids into monoglycerides and free fatty acids. Micelles, which are formed from monglycerides, fatty acids, cholesterol, bile salts and fat soluble vitamins, are absorbed by jejunal enterocytes, where they are degraded.(4) Chylomicra are synthesized within enterocytes from triglycerides (formed from glycerol and fatty acids), cholesterol esters, cholesterol, phospholipid and apolipoprotein. These chylomicra are then secreted into intestinal lacteals, and they eventually reach the plasma via lymphatics and the thoracic duct. Chylomicra are hydrolyzed by lipoprotein lipase to fatty acids and glycerol, which are absorbed by adipocytes/hepatocytes and stored as triglycerides.(4) Lipid is transported in plasma bound to apolipoprotein, which is synthesized and secreted by the liver. Lipoproteins are classified (with increasing density) as chylomicra, very low density (VLDL), intermediate density (IDL), low density (LDL) or high density (HDL) lipoprotein.(4)
Very low density lipoprotein is synthesized in the liver; in addition to triglyceride, cholesterol and phospholipid (in a ratio of 4:1:1), it has three apolipoproteins: B-100, C and E. ApoC and ApoE are acquired from HDL. The primary function of VLDL is to deliver triglycerides to adipose tissue and striated muscle.(4,13) Within the capillaries of these tissues, VLDL is cleaved by lipoprotein lipase, producing IDL, which retains ApoE and ApoB-100; IDL can then follow two possible pathways: 1) the hepatocyte LDL receptor recognizes ApoB-100 or ApoE, allowing hepatic uptake and recycling to form VLDL, or 2) conversion to LDL by hepatic lipase (in hepatic sinusoidal capillaries) with removal of ApoE and most of the triglyceride. The LDL can then be cleared by the liver via LDL receptor recognition of ApoB-100, or it can be cleared by other methods, especially the monocyte/macrophage system, which contributes to the pathogenesis of atherosclerosis.(4,7,13) Defects in the LDL receptor, such as the inherited LDL receptor defect in Watanabe rabbits or in people with familial hypercholesterolemia,1 result in increased monocyte/macrophage-mediated clearance of excess LDLs, promoting the formation of xanthogranulomas and atherosclerosis.
Hepatic LDL receptor binding occurs within a clathrin-coated pit where LDL is internalized within a vesicle that fuses with the lysosome, resulting in degradation of ApoB-100 to its constituent amino acids, and the catabolism of cholesterol esters into free cholesterol.(7) The LDL receptor is subsequently recycled to the surface, while free cholesterol exits the lysosome via a process mediated by NPC1 and NPC2. This free cholesterol plays several roles in lipid metabolism. It can be utilized to form cell membrane components, steroid hormones and/or bile salts; it also activates acyl-coenzyme A, which leads to esterification and storage of excess cholesterol.(7) Free cholesterol within hepatocyte cytoplasm also inhibits LDL receptor synthesis, and inhibits HMG CoA reductase, which is the rate-limiting enzyme in cholesterol synthesis.(7)
In humans most cholesterol is transported as LDL, the plasma concentration of which is the most important risk factor for development of atherosclerosis.(14) The lipoprotein profile of swine bears the closest resemblance to that of humans, while dogs and cats have a higher proportion of HDL; as a result dogs and cats are generally considered to be resistant to atherosclerosis.(4) The exact pathogenesis of atherosclerosis in association with canine hypothyroidism has not yet been characterized.(14)
New data suggest an important role for chemokines and chemokine receptors in atherosclerosis,(13) and a recent study showed that advanced glycation end products (AGEs) may play a role in the pathogenesis of atherosclerosis in humans and dogs. Advanced glycation end products belong to group of compounds resulting from glycation and oxidation of proteins, lipids and nucleic acids; specifically, hyperglycemia and oxidant stress contribute to increased formation of AGEs.(3) In humans and dogs, deposition of AGEs has been demonstrated immunohistochemically in atherosclerotic lesions and it has been suggested that AGEs may contribute to the development of atherosclerosis via effects on lipoproteins, extracellular matrix proteins, inflammatory mediators, smooth muscle and vascular endothelial cells.(3) Advanced glycation end products may also suppress cellular antioxidants and induce expression of two important oxidized LDL (OxLDL) receptors: macrophage scavenger receptors class A and CD36. Increased expression of these receptors leads to enhanced uptake of OxLDLs, resulting in the transformation of macrophages to foam cells.(3)
Rule-outs for canine atherosclerosis include arteriosclerosis (i.e., age related, often incidental intimal fibrosis of large elastic arteries), arterial calcification (e.g., due to renal disease, vitamin D toxicosis, ingestion of calcinogenic plants or infectious diseases) or lysosomal storage diseases such as Mucopolysaccharidosis-I (MPS-I). Mucopolysaccharidosis-I is an inherited, autosomal recessive deficiency of α-L-iduronidase that causes lysosomal accumulation of glycosaminoglycans (GAGs) in a variety of cell types, including those within the vascular system (see WSC 2013-14, conference 5, case 2).(9) A similar disease (i.e., Hurler/Scheie syndrome) affects humans.(7) Some dogs with MPS-I develop occlusive plaques affecting the tunica intima of medium to large arteries near branch points, or areas of low shear stress, such as the distal aorta or coronary and mesenteric arteries.(9) Histologically, intimal plaques consist of abundant extracellular matrix admixed with foamy (i.e., GAG-laden) macrophages, fibroblasts and smooth muscle cells.(9)
References:
2. Beaufrere H, Nevarez JG, Wakamatsu N, Clubb S, Cray C, Tully TN. Experimental dietinduced atherosclerosis in Quaker parrots (Myiopsitta monachus). Vet Pathol. 2013;50(6):1116-1126.
3. Chiers K, Vandenberge V, Ducatelle R. Accumulation of advanced glycation end products in canine atherosclerosis. J Comp Pathol. 2010;143(1):65-69.
4. Evans EW. Proteins, lipids and carbohydrates. In: Latimer KS, ed. Duncan and Prasses Veterinary Laboratory Medicine Clinical Pathology. 5th ed. Ames, IA: John Wiley and Sons; 2011:183-191.
5. Hess RS, Kass PH, Van Winkle, TJ. Association between diabetes mellitus, hypothyroidism or hyperadrenocorticism, and atherosclerosis in dogs. J Vet Intern Med. 2003;17:489-494.
6. Kagawa Y, Hirayama H, Uchida E, Izumisawa Y, Yamaguchi M, Kotani T, et al. Systemic atherosclerosis in dogs: histopathological and immunohistochemical studies of atherosclerotic lesions. J Comp Path. 1998;118:195-206.
7. Kumar V, Abbas AK, Fausto N, Aster JC, eds. Genetic disorders. In: Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Saunders Elsevier; 2010:147-150.
8. Liu S, Tilley LP, Tappe JP, Fox PR. Clinical and pathologic findings in dogs with atherosclerosis: 21 cases (1970-1983). J Am Vet Med Assoc. 1986;2:227-232.
9. Lyons JA, Dickson PL, Wall JS, et al. Arterial pathology in canine mucopolysaccharidosis-I and response to therapy. Lab Invest. 2011;91(5):665-674.
10. Maxie MG, Robinson WF. Cardiovascular system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 5th ed. Vol 3. St. Louis, MO: Saunders Elsevier; 2007:57-59.
11. Miller LM, Van Vleet JF, Gal A. Cardiovascular system and lymphatic vessels. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:560-561.
12. Mitchell RN, Schoen FJ. Blood vessels. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Saunders Elsevier; 2010:496.
13. Schillinger L, Lemberger K, Chai N, Bourgeois A, Charpentier M. Atherosclerosis associated with pericardial effusion in a central bearded dragon (Pogona vitticeps). J Vet Diagn Invest. 2010;22:789-792.
14. Vitale CL, Olby NJ. Neurologic dysfunction in hypothyroid, hyperlipidemic Labrador retrievers. J Vet Intern Med. 2007;21(6):1316-1322.