Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 15
January 17th, 2018
CASE IV: 401343 (JPC 4103279).
Signalment: 4-year-old, female, Scottish blackface sheep, Ovis aries, ovine.
History: The animal was referred for ill thrift and respiratory distress.
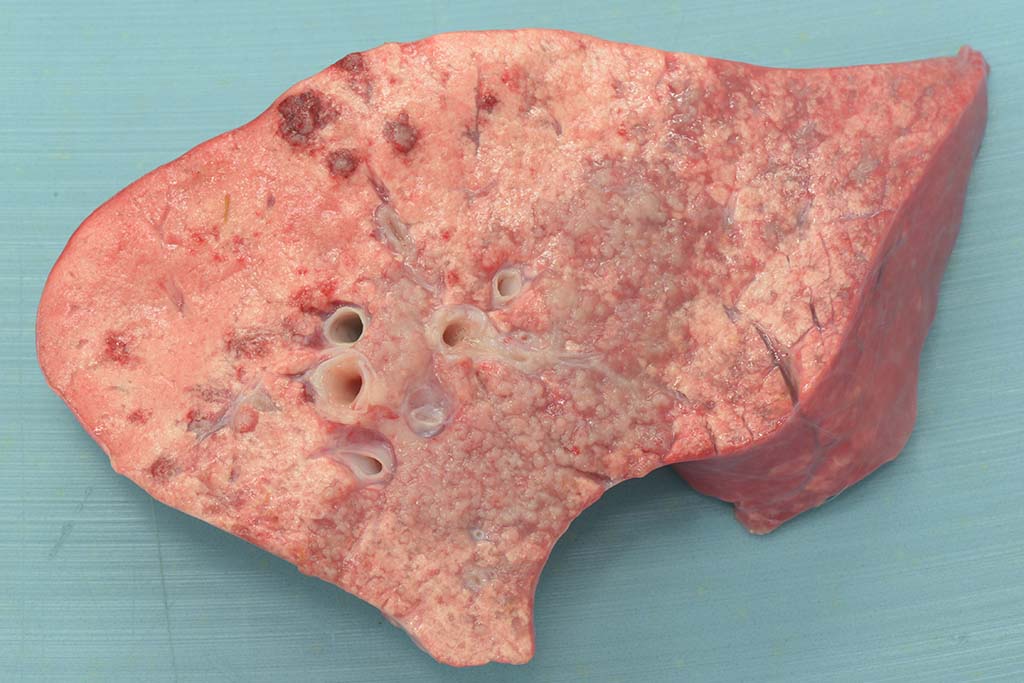
Gross Pathology: This animal is in poor body condition (BCS: 1.5/5). Small amounts of white froth are noted at the nares and within the lumen of the trachea. In a multifocal to coalescing distribution and affecting predominantly the right middle and caudal pulmonary lobes, the pulmonary parenchyma is effaced by multiple, reasonably well-demarcated, pale pink to grey, firm masses. On cut surface, small amounts of clear fluid run from the surface of the masses, which have a grey, granular appearance.
Laboratory results: None provided.
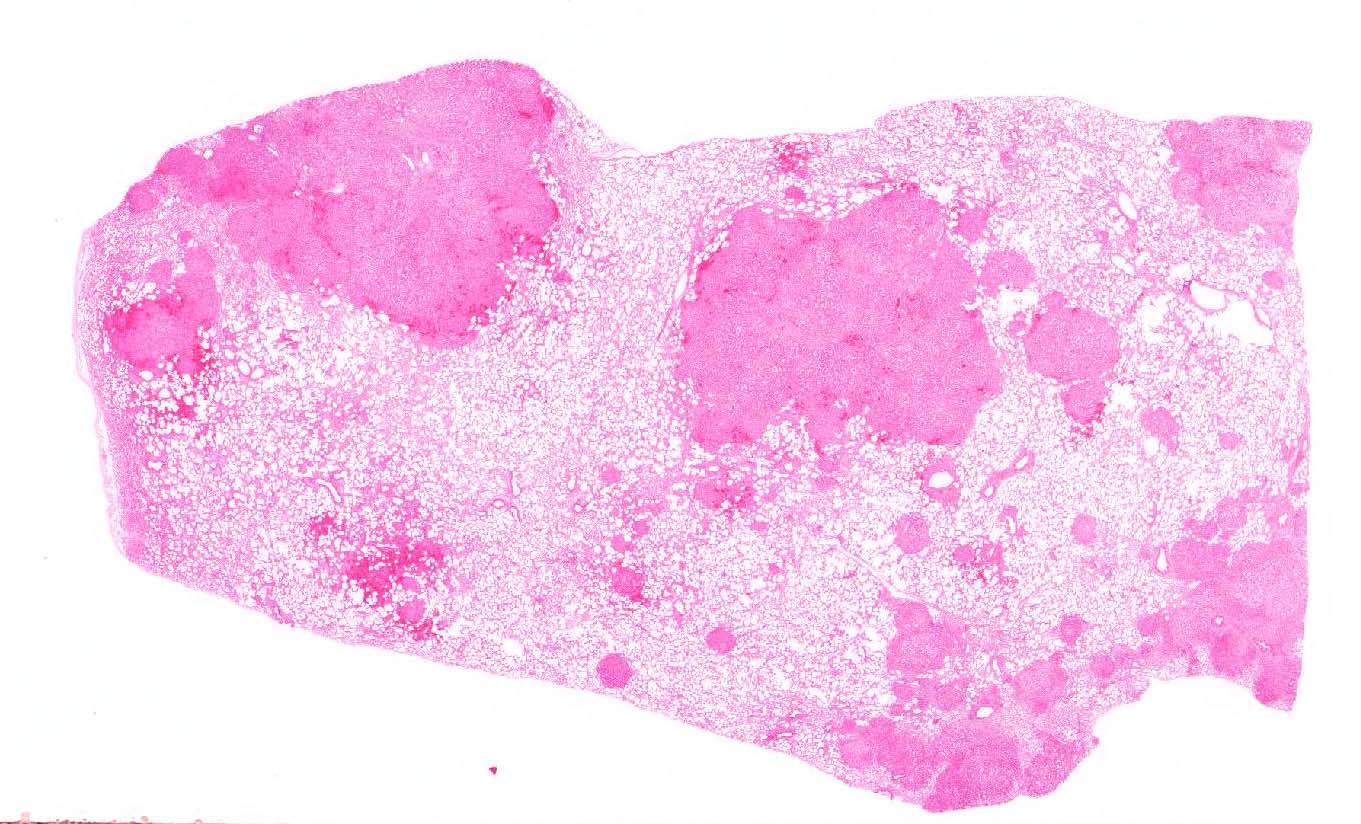
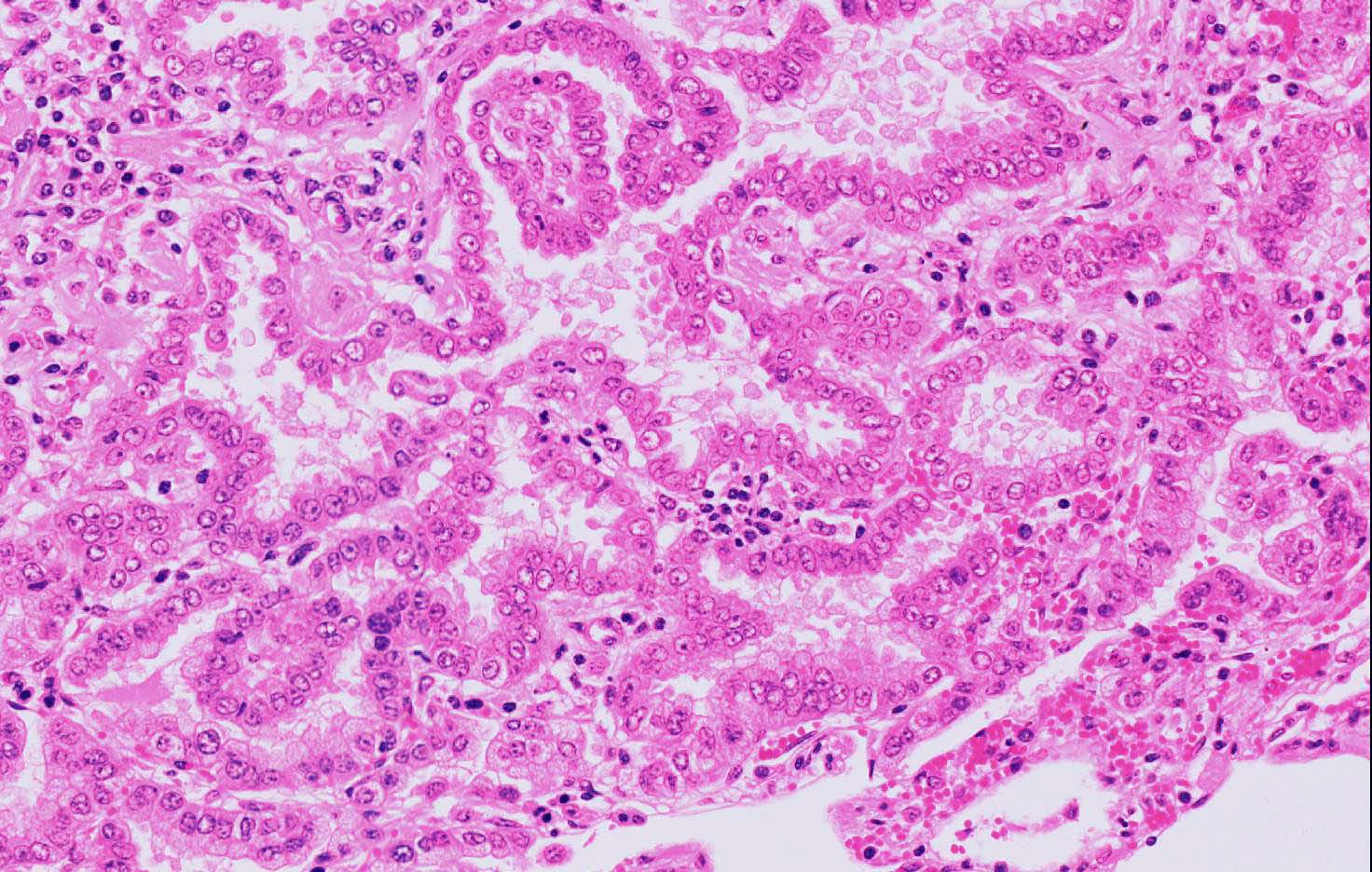
Microscopic Description: Lung: Affecting approximately 50% of the pulmonary parenchyma are multiple, well-demarcated, yet infiltrative, moderately to highly cellular, multifocal to coalescing neoplastic masses composed of epithelial cells arranged in a predominantly lepidic, rarely acinar or even papillary pattern, which are supported by small amounts of poorly to moderately cellular collagenous stroma. Lining the alveoli is an, in most cases, single layer of cuboidal to columnar neoplastic cells with distinct cell borders, moderate to large amounts of eosinophilic, often also vacuolated cytoplasm, a round to oval basal nucleus with ropey, clumped and vesiculated chromatin and a small, basophilic nucleolus. There is minimal to mild anisocytosis and anisokaryosis, and four mitotic figures are present in 10 HPF, some of which are bizarre. Multifocally present within the lumina of the neoplastic-lined alveolar spaces or acini are small to moderate amounts of pale eosinophilic material and small numbers of neutrophils, macrophages, occasional small to moderately sized accumulations of extravasated erythrocytes (hemorrhage) and sloughed epithelial cells. The supporting stroma multifocally exhibits mildly dispersed collagenous fibers (edema), small numbers of extravasated red blood cells (hemorrhage) and in a multifocal to coalescing distribution contains small to moderate numbers of lymphocytes, plasma cells and smaller numbers of neutrophils. The alveolar spaces in the periphery of the neoplastic nodules also contain moderate numbers of alveolar macrophages, smaller numbers of neutrophils, occasional lymphocytes and multifocally, moderate to large numbers of extravasated erythrocytes. Multifocally, surrounding the bronchioles are small to moderate numbers of lymphocytes and plasma cells in a multifocal to coalescing distribution.
Contributor’s Morphologic Diagnosis:
Lung: Pulmonary adenocarcinoma, multifocal to coalescing.
Contributor’s Comment: Ovine pulmonary adenocarcinoma (OPA; also known as Jaagsiekte or ovine pulmonary adenomatosis) is an infectious and contagious neoplastic disease caused by a beta retrovirus, Jaagsiekte sheep retrovirus (JSRV), an enveloped RNA virus that primarily targets sheep but also, rarely goats. This disease is present worldwide, and is particularly common in South America, South Africa and Scotland with certain breeds of sheep exhibiting a predisposition. It is absent in Australia and New Zealand and has been eradicated in Iceland.5,9
Affected animals typically present with progressive dyspnea, tachypnea, nasal discharge, coughing and weight loss.5 A characteristic feature of this disease is the production of excessive amounts of frothy to milky fluid (surfactant proteins) from the nostrils especially when the head is lowered (wheelbarrow test), although it has been reported the amounts of fluid produced can be variable between individuals.7
Multifocal or locally extensive neoplasms are found in the pulmonary parenchyma with metastases to the regional lymph nodes occurring in approximately 0.3-25% of cases. More distant metastases are rare but when present, they can spread to different organs with in order of frequency: liver, kidneys, skeletal muscle, digestive tract, spleen, skin and adrenal glands.11
JRSV induces oncogenic transformation of type II pneumocytes, Clara cells and progenitor cells of the pulmonary airway epithelia8,9, however the exact pathogenic mechanisms of viral neoplastic transformation are poorly understood. It has been reported that attachment of the virus to the host cell is mediated through the binding of the SU subunit of the viral Env protein (envelope protein) to a specific cell surface receptor, Hyal2 on the host cell9,12, which in turn mediates the entry of the virus into the cell via endocytosis.3 As with all RNA viruses, reverse transcription, where the single-stranded RNA genome is converted into a double-stranded, DNA genome, then takes place within the cytoplasm, which is essential for integration of the virus into the host genome.9 The Env protein has been shown to be able to induce similar tumors in immunodeficient mice and has also been shown to induce oncogenesis in rat fibroblasts, however as stated previously, the mechanisms are not clearly known.12
Spread of JRSV between sheep occurs through respiratory secretions from affected sheep. Additionally, lambs can be infected through their dams from the ingestion of infected colostrum.4
Two forms of OPA have been described, the classical and the atypical form. In the classical form, sheep present with the clinical signs as described above and typically contain multifocal to coalescing neoplastic lesions in the cranioventral pulmonary lung lobes. Grossly, the lungs are heavy, wet and fail to collapse. On cut surface, the tumors often have a grey, granular appearance and large amounts of fluid run from the surface. In contrast, the atypical form often follows a subclinical course and grossly, neoplasms are seen in the diaphragmatic lung lobes. Tumors in the atypical form are dry, white and have a multifocal distribution.9,11
Microscopically, the histopathological features of both the classic and the atypical form are similar with several patterns including the lepidic pattern where the alveoli are lined by either cuboidal or columnar neoplastic cells. Other patterns include the papillary and acinar patterns.5,9 The atypical form differs from the classical one by having more well-demarcated neoplastic lesions which are associated with larger numbers of mononuclear cells, in particular CD4 and CD8 T-cell subsets, as well as increased amounts of fibrous tissue. It has been suggested that infiltration of mononuclear cells into the tumors of atypical cases may be due to an immunological response to tumorigenesis, whereas in the classical form, there is suppression of such infiltration.2,13 Neoplastic processes can be complicated by the presence of concurrent infections including Maedi-Visna, a viral infection caused by a non-oncogenic retrovirus and resulting in interstitial pneumonia with the formation of prominent lymphoid nodules as well as interstitial fibrosis and hypertrophy of smooth muscle. In addition, bacterial infections are prevalent in a large number of sheep affected by OPA, such as those caused by Mycoplasma spp, and may contribute to death of the animal.6,9
Although rare, non-viral pulmonary carcinomas are difficult to distinguish from ovine pulmonary adenocarcinoma based on the gross and histological lesions alone and immunohistochemistry is required to detect the JSRV envelope glycoprotein for definitive confirmation.5
Clinically, a further viral-induced epithelial tumor, however present in the nasal cavity, can produce similar clinical signs of increased fluid production from the nostrils. The enzootic nasal tumor is also caused by a retrovirus, the enzootic nasal tumor virus type 1 (ENTV-1) in sheep and the enzootic nasal tumor virus type 2 (ENTV-2) in goats, which infects the secretory epithelial cells of the nasal glands and here leads to the formation of nasal epithelial masses with abundant secretion of mucus.9,14
JPC Diagnosis: Lung: Pulmonary adenocarcinoma, Scottish blackface sheep (Ovis aries), ovine.
Conference Comment: Jaagsiekte sheep retrovirus (JSRV) belongs to the family Retrovirdae, subfamily Orthoretrovirinae, and genus Betaretrovirus. JSRV (as described above) is the causative agent of contagious lung tumors in sheep called ovine pulmonary adenocarcinoma. The term “jaagsiekte” is taken from the Afrikaans words for “chase” (jag) and “sickness” (siekte) describing the common clinical scenario of sheep that exhibit respiratory distress from being chased. The infectious “exogenous” form of JRSV has an endogenous counterpart (enJRSVs) which is present in the genome of healthy sheep and goats.15 Sheep have approximately 27 copies of enJRSVs that are able to block the JSRV replication cycle and have an essential role in fetal development and formation of the placenta.1
JRSV is transmitted via respiratory droplets and infect respiratory epithelial cells (type II pneumocytes or Clubb cells) as well as lymphocytes and myeloid cells, but replicates most in alveolar type II pneumocytes and Club cells.10 This virus has the typical “gag”, “pol”, and “env” genome arrangement bordered on each end by a long terminal repeat (LTR). The “gag” portion encodes the internal structural matrix, capsid, and nucleocapsid proteins. “Pol” encodes the RT and integrase enzymes. Most importantly, “env” encodes surface and transmembrane envelope glycoproteins which aid in viral cellular entry and directly stimulate neoplastic transformation of type II pneumocytes and/or Club cells.9
An interesting feature of JRSV infection is lack of host immune response. There are two possible explanations for this phenomenon. (1) The sheep may be immunologically tolerant to JRSV due to the already present enJRSV that would be expressed in the fetal thymus during T-cell development. Thus, any anti-JRSV reactive T-cells would be removed via self-tolerance mechanisms. (2) Another possibility is that tumor cells downregulate their expression of MHC-I. This hypothesis is supported by research that has identified an absence of virus-specific cytotoxic T-cells.9
Contributing Institution:
Division of Pathology, Public Health and Disease Investigation
Veterinary Diagnostic Services
School of Veterinary Medicine
College of Medical, Veterinary and Life Sciences
University of Glasgow (Garscube Campus)
464 Bearsden Road
Glasgow G61 1QH, Scotland
http://www.gla.ac.uk/schools/vet/
References:
- Arnaud F, Varela M, Spencer TE, Palmarini M. Coevolution of endogenous betaretroviruses of sheep and their host. Mol. Life Sci. 2008;65(21):3422–3432.
- Azizi S, Tajbakhsh E, Fathi F. Ovine pulmonary adenocarcinoma in slaughtered sheep: A pathological and polymerase chain reaction study. J S Afr Vet Assoc. 2014;85:932.
- Bertrand P, Cote M, Zheng Y-M, et al. Jaagsiekte sheep retrovirus utilizes a pH-dependent endocytosis pathway for entry. J Virol. 2008;82:2555-2559.
- Borobia M, De las Heras M, Ramos JJ, et al. Jaagsiekte sheep retrovirus can reach Peyer’s patches and mesenteric lymph nodes of lambs nursed by infected mothers. Vet Pathol. 2016;53:1172-1179.
- Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 6th Vol. 2: St. Louis, MO: Elsevier Ltd; 2016:560-562.
- Cousens C, Gibson L, Finlayson J, et al. Prevalence of ovine pulmonary adenocarcinoma (Jaagsiekte) in a UK slaughterhouse. Vet Rec. 2015;176:413.
- Cousens C, Thonur L, Imlach S, et al. Jaagsiekte sheep retrovirus is present at high concentration in lung fluid produced by ovine pulmonary adenocarcinoma-affected sheep and can survive for several weeks at ambient temperature. Res Vet Sci. 2009;87:154-156.
- De las Heras M, de Martino A, Borobia M, et al. Solitary tumours associated with Jaagsiekte retrovirus in sheep are heterogenous and contain cells expressing markers identifying progenitor cells in lung repair. J Comp Pathol. 2014;150:138-147.
- Griffiths DJ, Martineau HM, Cousens C. Pathology and pathogenesis of ovine pulmonary adenocarcinoma. J Comp Pathol. 2010;142:260-283.
- Lopez A, Martinson SA. The respiratory system. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, MO: Elsevier;2016:553-554.
- Minguijon E, Gonzalez L, De las Heras M, et al. Pathological and aetiological studies in sheep exhibiting extrathoracic metastases of ovine pulmonary adenocarcinoma (Jaagsiekte). J Comp Pathol. 2013;148:139-147.
- Rai SK, Duh FM, Vigdorovich V, et al. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for Jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci USA. 2001;98:4443-4448.
- Summers C, Benito A, Ortin A, et al. The distribution of immune cells in the lungs of classical and atypical ovine pulmonary adenocarcinoma. Vet Immunol Immunopathol. 2012;146:1-7.
- Walsh SR, Linnerth-Petrik NM, Yu DL, et al. Experimental transmission of enzootic nasal adenocarcinoma in sheep. Vet Res. 2013;44:66.
- York DF, Querat G. A history of ovine pulmonary adenocarcinoma (jaagsiekte) and experiments leading to the deduction of the JSRV nucleotide sequence. Top. Microbiol. Immunol. 2003;275:1–23.