CASE 4: T2813/19 11 (4140288-00)
Signalment:
Finishing pig, male, 7-8 month, SKS line, Sus scrofa
History:
This pig was part of an ?in field?-vaccination study against Mycoplasma hyopneumoniae and porcine circovirus type 2 (PCV2). The clinical examination revealed respiratory symptoms typical for M. hyopneumoniae, like dry and non-productive coughing. To confirm this suspected diagnosis the lungs were examined grossly at the abattoir. The determined lung score, using the scheme of Madec and Kobisch (5), with values up to 20 confirmed the clinical diagnosis.
Gross Pathology:
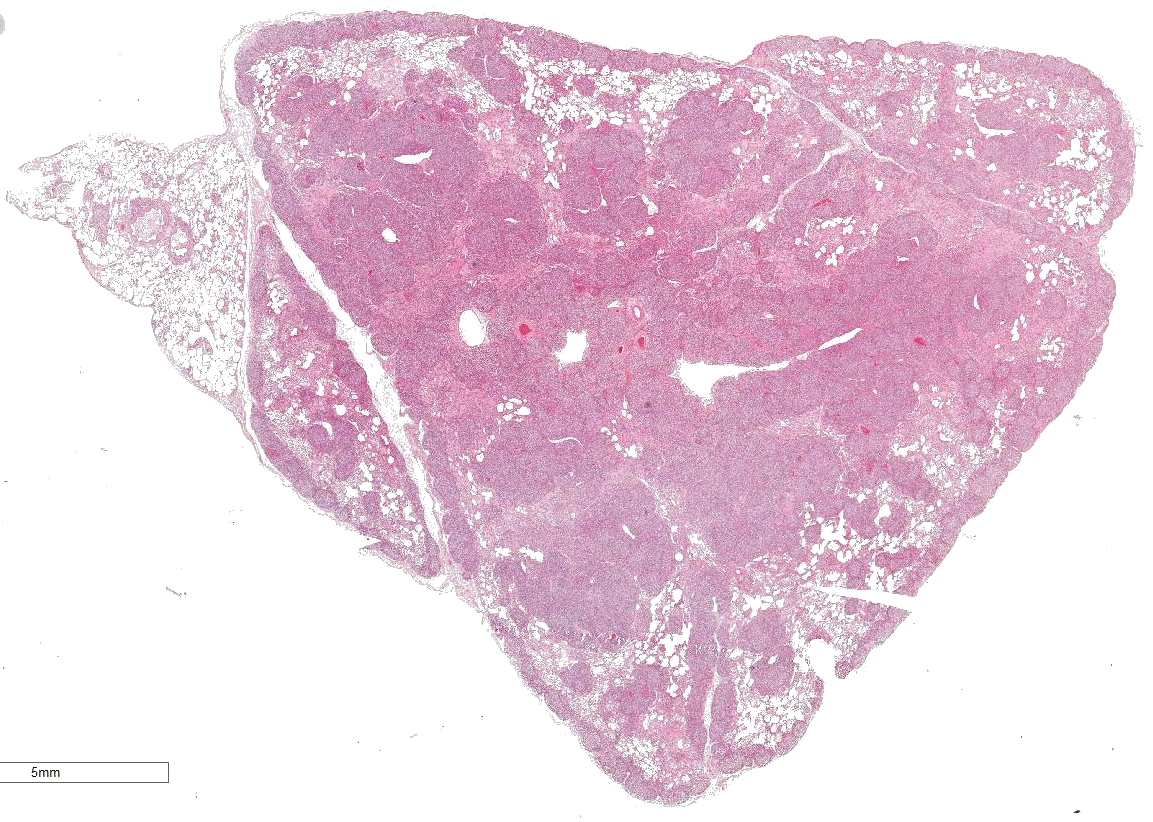
The tissue collected at the abattoir showed cranioventral consolidated lungs with lesions consistent with catarrhal and suppurative bronchopneumonia.
Laboratory results (PCR/RT-PCR):
PCV2 (DNA): negative
PRRSV (RNA) (EU+US): negative
M. hyopneumoniae (DNA): positive
A. pleuropneumoniae (DNA): negative
H. parasuis (DNA): negative
Swine influenza Virus (RNA): negative
Microscopic description:
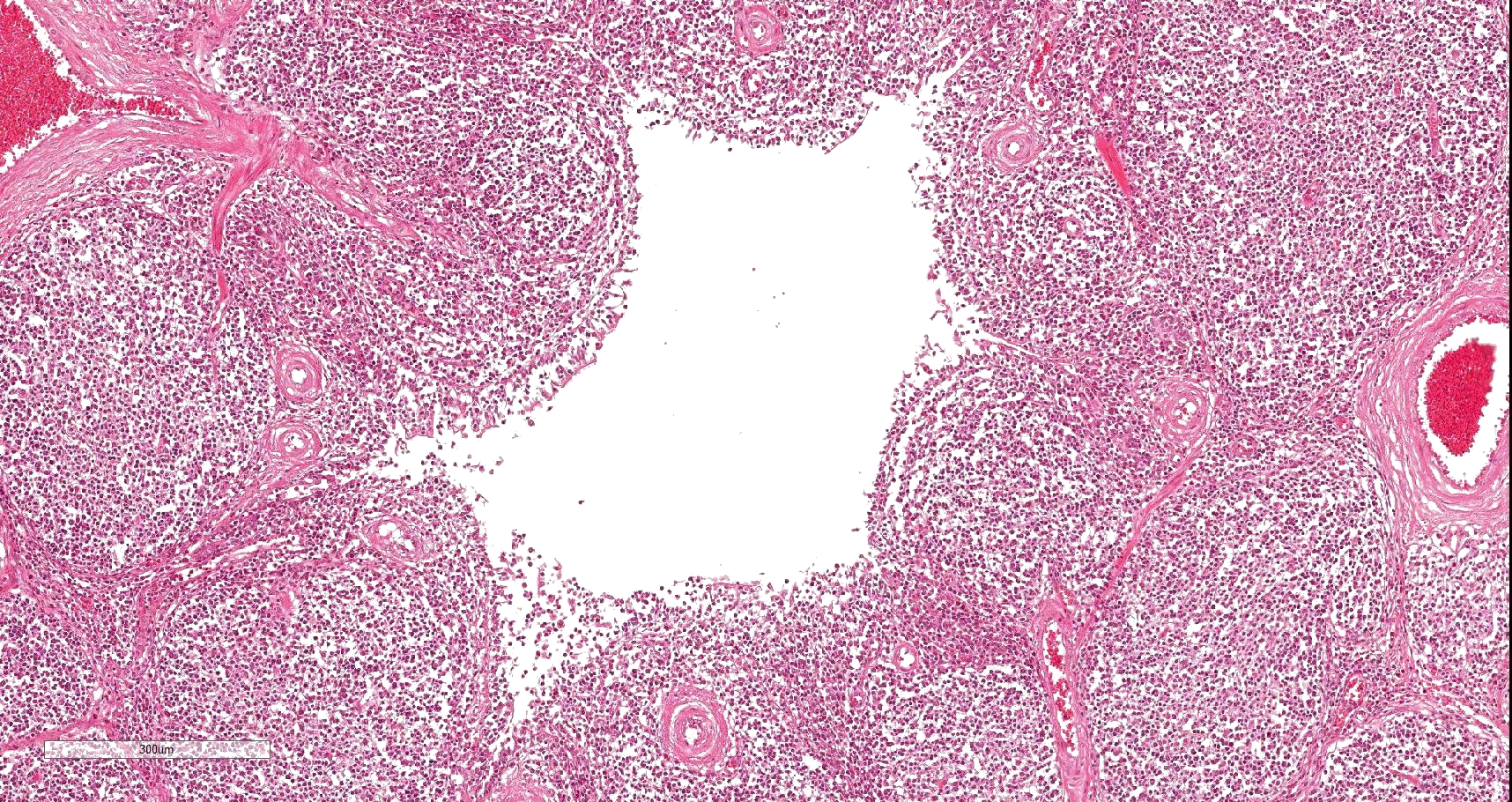
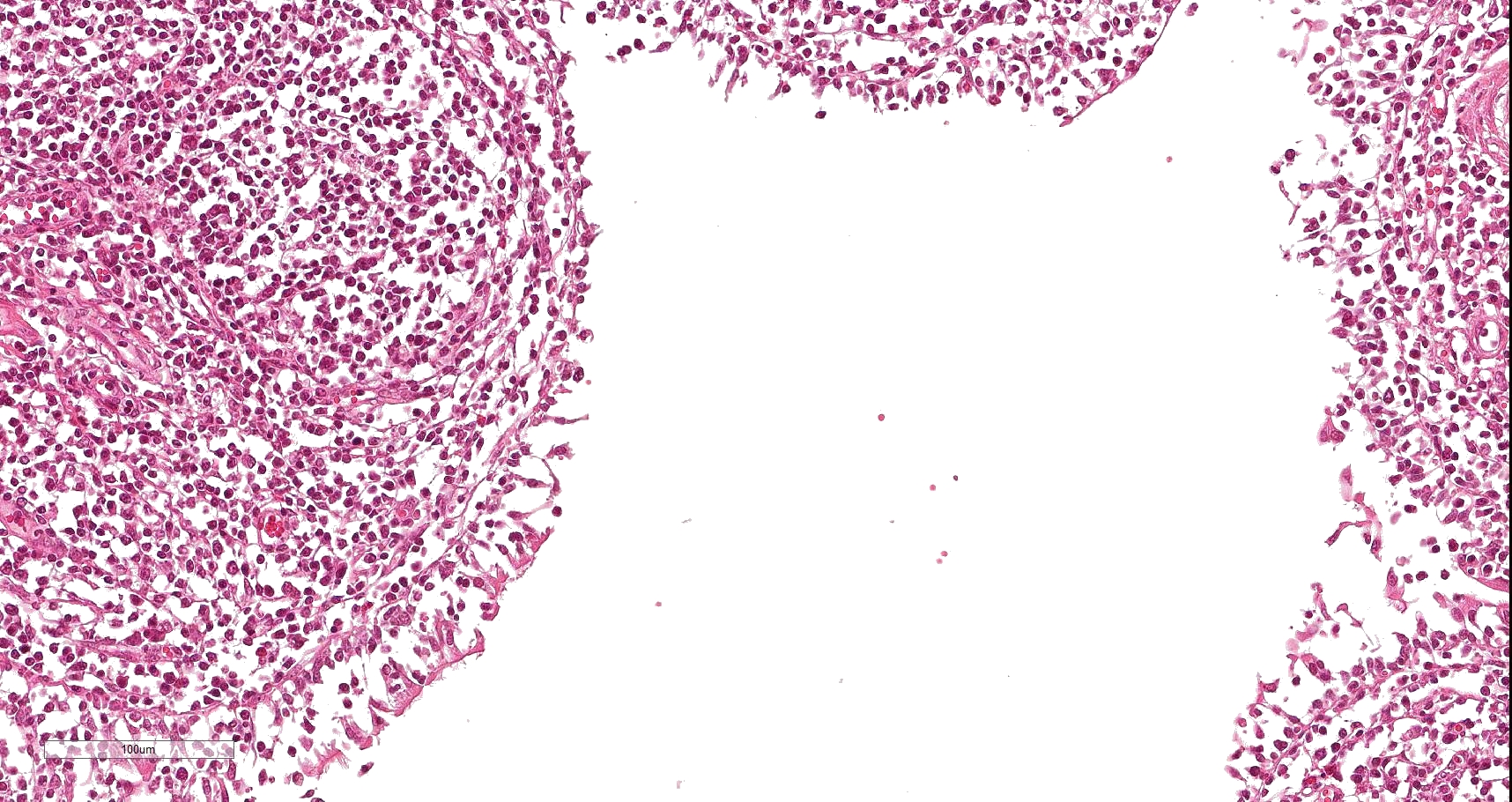
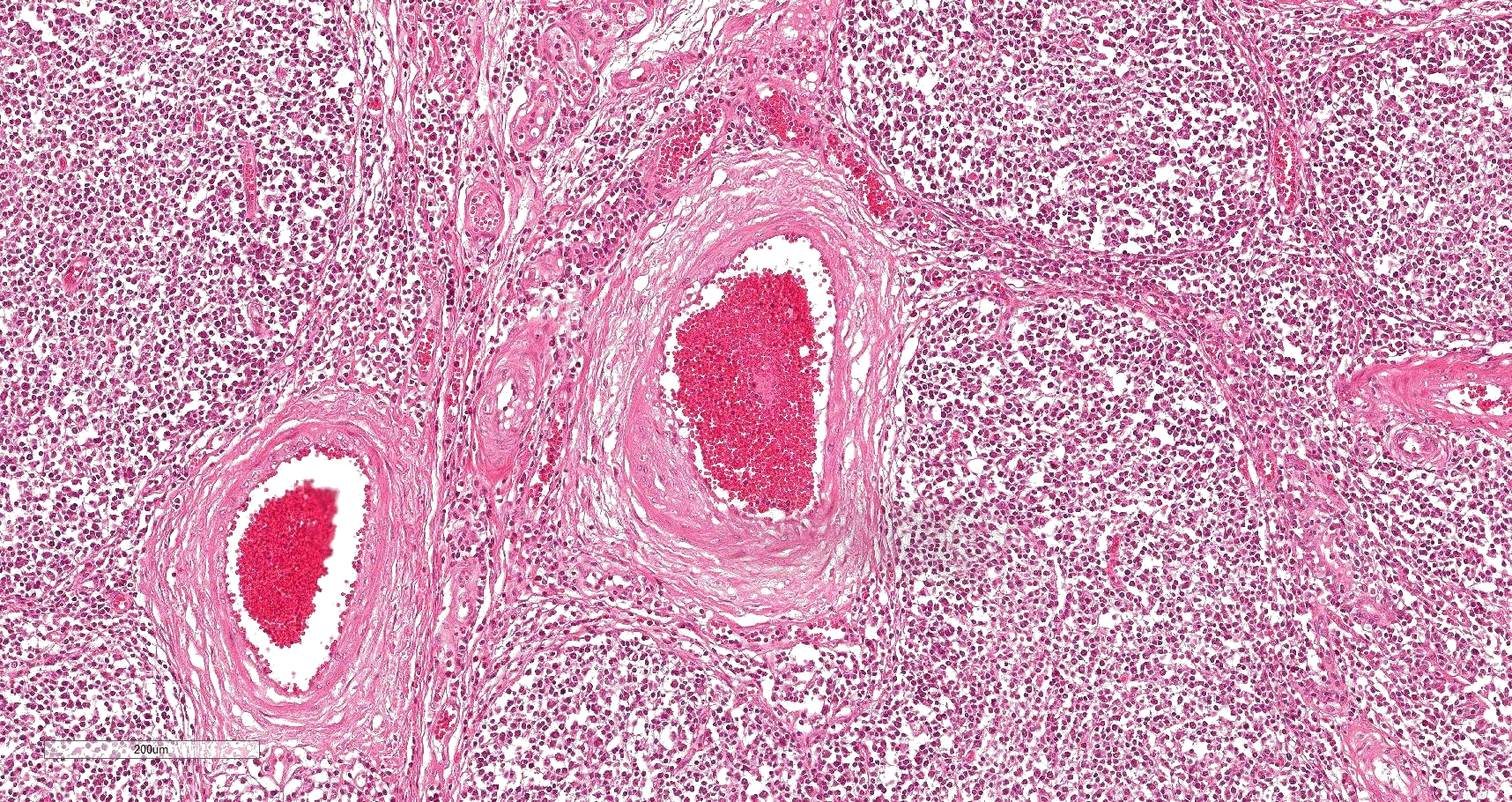
Lung: Affecting 80 % of the tissue, there is a marked follicular hyperplasia of peribronchiolar, peribronchial, and perivascular lymphoid tissue compressing, infiltrating and effacing the structure of the adjacent alveoli. Often follicles line up beneath the pleura. The follicles are characterized by a central paler zone of lymphocytes (germinal center) rimmed by a zone of more densely packed lymphocytes (mantle zone). Adjacent alveoli are either compressed (atelectasis) or expanded by a mixture of homogenous eosinophilic fluid (edema) admixed with neutrophils, macrophages, lymphocytes and plasma cells, and few erythrocytes. Alveolar septa are expanded by lymphocytes and plasma cells. Bronchi and bronchioles often have attenuated (sometimes lost), cuboidal epithelium with loss of cilia. Lumina frequently contain sloughed epithelial cells admixed with degenerate inflammatory cells. There is mild edema of the interlobular septa with infiltration of low numbers of neutrophils, macrophages, lymphocytes and plasma cells.
Immunohistochemistry
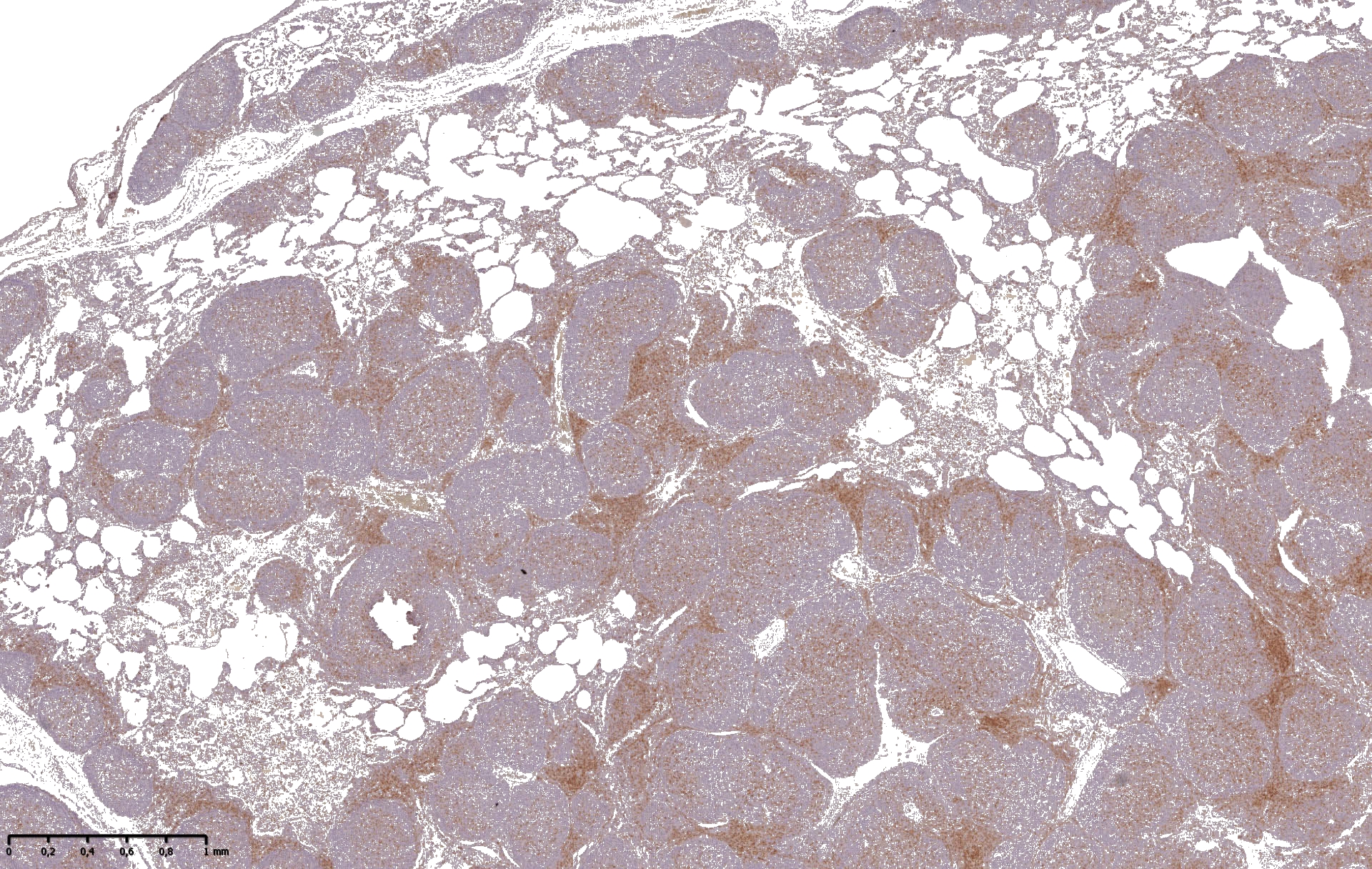
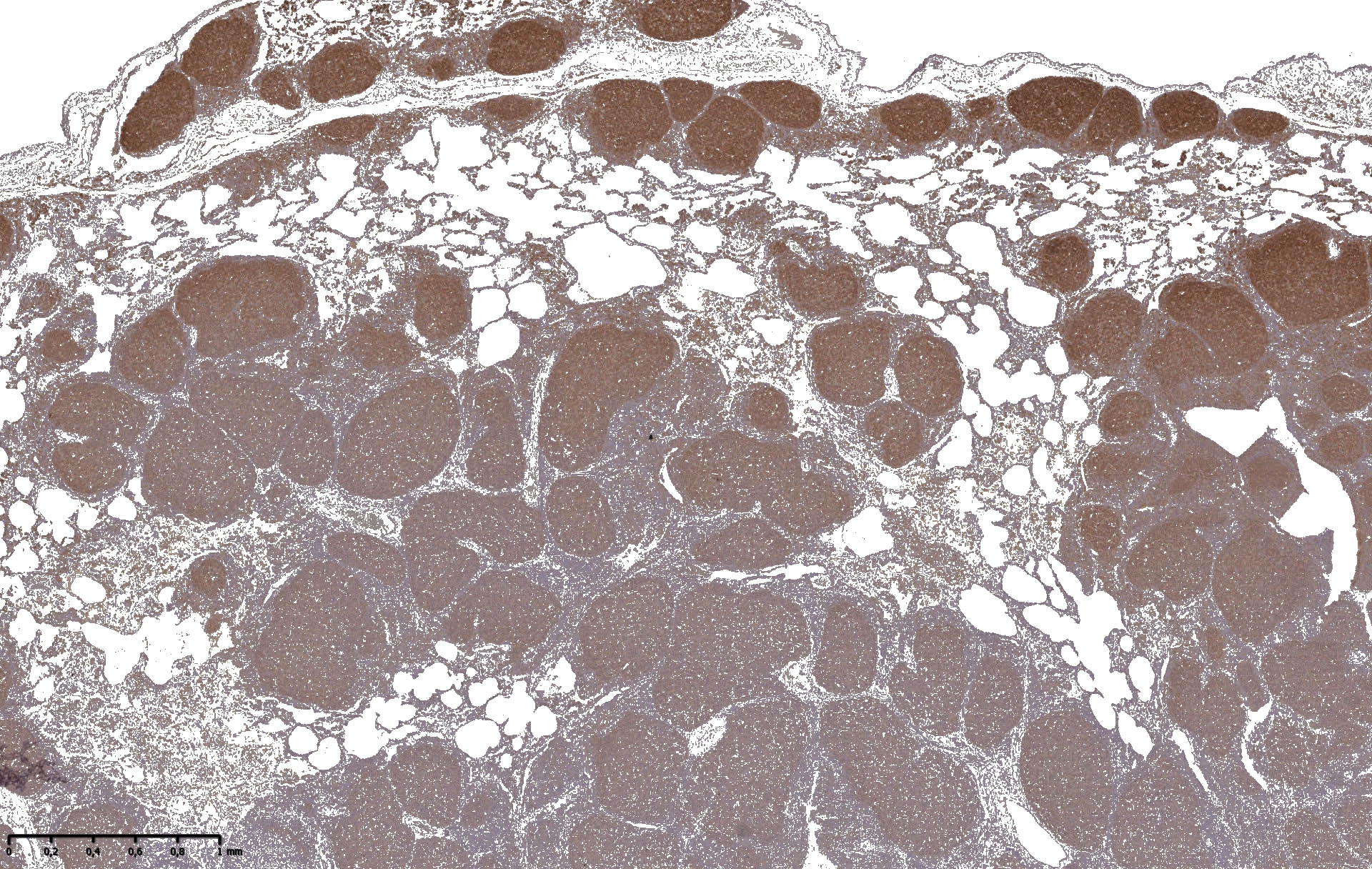
CD3/CD20 - for lymphocyte characterization
Contributor's morphologic diagnosis:
Lung: Pneumonia, bronchointerstitial, lympho-histiocytic, multifocal to coalescing, chronic, severe with marked lymphoid hyperplasia; Bronchopneumonia, catarrhalic, multifocal to coalescing, chronic, moderate. Fattening pig. Sus scrofa.
Contributor's comment:
Enzootic pneumonia (EP) caused by Mycoplasma hyopneumoniae is a common chronic lung disease of pigs. EP is the most important lung disease in grower-finisher pigs worldwide and causes major economic losses to the pig industry. The course of the disease progresses over weeks with low mortality, but high morbidity (up to 100%). Coughing, reduced weight gain and reduced feed conversion are the main clinical signs.1,5 Furthermore, the pathogen is a primary agent of the porcine respiratory disease complex (PRDC) interacting with other porcine pathogens (e.g. PRRSV, PCV2, Swine influenza virus, Pasteurella multocida, Actinobacillus pleuropneumoniae, Haemophilus parasuis, Bordetella bronchiseptica). Other influencing factors are housing conditions and management practices (like crowding, poor air quality, stress). The result is a more severe multifactorial disease.10
Mycoplasma hyopneumoniae, like other mycoplasma species, is pleomorphic, lacks a cell membrane and has a small genome. Dams and piglets often serve as reservoirs. The most important way of transmission is horizontal. Vectors do not play an important role, but airborne transmission has been suspected.1,5,7 The incubation period is variable (1-6 weeks post infection) and the ciliated epithelium of the respiratory tract is the first target using different adhesins (e.g. P97, P102, P159). Mycoplasmas lack classical virulence factors like toxins, but they can use glycerol as a carbon source and the production of hydrogen peroxide can be triggered.7
Lungs are affected cranioventrally along the bronchioles, showing purple to gray consolidated areas with atelectasis and a rubbery or thymus like texture of the parenchyma. Histopathologically, inflammatory infiltrates are centered around the airways and expand into the interstitium, with extensive lymphoid hyperplasia and increased number of alveolar macrophages. Additionally, goblet cell hyperplasia, bronchial gland hyperplasia, alveolar and interstitial edema, loss of ciliated cells, and epithelial hyperplasia can be detected.1,5
Cell membrane proteins of Mycoplasma sp. are prone to serve as superantigens, leading to polyclonal lymphocyte proliferation.1 Immune evasion of the mycoplasmas and the immune response of the host are considered to be the main driver of pulmonary lesions in EP. Inflammation is triggered by different cytokines like IL-1, TNF, and IL-6 as well as by interactions of Mycoplasma adhesin molecules with the host?s plasminogen.7
Immunohistochemically, lymphocytes in the affected tissue are composed of numerous follicle forming-B cells (CD20) and lower numbers of perifollicular T cells (CD3) (see figs.). This finding highlights the importance of the pathogen/immune system interaction for the pathogenesis of EP.
In the presented case lymphoid hyperplasia is remarkable with extensive loss of respiratory epithelium and loss of normal tissue architecture, characterizing the chronicity of the infection. Hyperplasia is so marked, that it may even resemble lymphoid neoplasia histologically. It can be suspected that the animals were exposed to virulent strains of Mycoplasma hyopneumoniae for longer periods and that the vaccination protocol in this group (a significant number of animals showed similar lesions) did not sufficiently control the infection.
Detection of Mycoplasma hyopneumoniae is possible by different established methods, including immunohistochemistry, in-situ hybridization or PCR. Due to the special growth requirements of the pathogen bacterial culture is not a useful method.6,7,10 In the presented case other relevant pathogens were excluded by PCR.
Vaccination is able to reduce clinical signs and lung lesions, and thereby improves performance. A reduction of the number of infectious organisms on the respiratory epithelium can be observed.6,7 Different vaccination protocols exist depending on the type of herd, management and production system, and infection pattern. The most common strategy is single or two-dose application. The antigens of different pathogens often need to be combined to achieve the best beneficial effect of vaccinations.
Nevertheless, there are still knowledge gaps, especially with regard to the interaction of Mycoplasma hyopneumoniae with the respiratory tract. Therefore, further research is necessary, for a better understanding, control and elimination of the pathogen.
Contributing Institution:
Institut fuer Veterinaer-Pathologie, Justus-Liebig-Universitaet Giessen
Frankfurter Str. 96, 35392 Giessen, Germany
http://www.uni-giessen.de/cms/fbz/fb10/institute_klinikum/institute/pathologie
JPC diagnosis:
Lung: Pneumonia, bronchointerstitial, lympho-histiocytic, diffuse, severe, with marked lymphoid hyperplasia.
JPC comment:
The contributor provides a good summary of enzootic pneumonia of swine. There are numerous adjuvanted, inactivated, whole-cell preparation vaccines used in swine-producing countries, and live-attenuated vaccines available in China and Mexico. Unfortunately, these vaccines do not provide complete protection, and further elucidation of M. hyopneumoniae?s pathogenesis and virulence factors may improve the effectiveness of new vaccines. Virulence factors such as surface lipoproteins, surface aminopeptidases, and hydrogen peroxide production have been investigated. More recently, the ability to evade host defenses has received increased attention. Gentamicin survival experiments performed on immortalized porcine alveolar macrophages (cell line PAM 3D4/21) which showed that very few M. hyopneumonia organisms are phagocytosed by alveolar macrophages as compared to a control E. coli strain, suggesting the ability to evade phagocytosis. Opsonization with anti-M. hyopneumoniae antibody did not promote increased phagocytosis, which may help explain the lack of efficacy of current vaccines.2
While vaccines are used, the other arm of treatment of M. hyopneumoniae infection is antibiotic therapy. As with testing of any kind across multiple labs in different locations, worldwide antibiotic susceptibility testing is problematic. When testing is performed on different equipment (let alone different brand equipment), under different conditions, by different personnel, for different populations, the results are not necessarily transportable. In each testing in the distributed model, there may be effector modifiers, known or unknown, that can lead to variation in test results. When trying to apply a result to a new population, it is often not possible to know whether conditions are sufficiently similar as for the result to be valid in the new population.3 However, the MycoPath program in Europe aims to improve some aspects of transportability by performing antibiotic susceptibility testing of numerous strains of M. hyopneumonia and M. bovis in a single lab under controlled settings. In this testing program, strains of M. hyopneumoniae from Belgium, Spain, and the United Kingdom were collected from national laboratories and forwarded to the MycoPath central laboratory in Brussels, Belgium for testing. Appropriate caution should be used in the attempt to apply these results to new populations. However, one clear benefit of the testing highlighted the differences in MIC between M. hyopneumonia and M. bovis strains.4
Similar to M. bovis, further elucidation of virulence factors will aid our understanding of these pathogens. Currently, it has been shown that M. hyopneumoniae are capable of entering and surviving within vesicle-like structures in endothelial cells of the respiratory tract. They make contact with fibronectin and integrin B1 receptors on the epithelial cell surface and are engulfed and enter the cell through clathrin-coated pits and caveolae-mediated endocytosis. Within the cell, this diminutive bacterium then induces cytoskeleton rearrangements within epithelial cells, causing significant pathology to the respiratory tract.9
References:
- Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 6th ed. Vol . Elsevier, St. Louis, Missouri; 2015:465-891.
- Deeney AS, Maglennon GA, Chapat L, Crussard S, Jolivet E, Rycroft AN. Mycoplasma hyopneumoniae evads phagocytic uptake by porcine alveolar macrophages in vitro. Veterinary Research. 2019;50:51.
3. Institute of Medicine. Ethical and Scientific Issues in Studying the Safety of Approved Drugs. 2019. Washington, DC: The National Academies Press. https://doi.org/10.17226/13219.
- Klein U, de Jong A, Moyaert H, et al. Antimicrobial susceptibility monitoring of Mycoplasma hyopneumonia and Mycoplasma bovis isolated in Europe. Veterinary Microbiology. 2017;204:188-193.
- Lopez A, Martinson SA. Respiratory system, mediastinum, and pleurae. In: Zachary JF, ed. Pathologic basis of veterinary disease. 6th ed. Elsevier, St. Louis, Missouri; 2017:471-560.
- Maes D, Segales J, Meyns T, Sibila M, Pieters M, Haesebrouck F. Control of Mycoplasma hyopneumoniae infections in pigs. Vet Microbiol. 2008;126(4):297-309.
- Maes D, Sibila M, Kuhnert P, Segalés J, Haesebrouck F, Pieters M. Update on Mycoplasma hyopneumoniae infections in pigs: Knowledge gaps for improved disease control. Transbound Emerg Dis. 2018;65(Suppl.1):110-124.
- Ostanello F, Dottori M, Gusmara C, Leotti G, Sala V. Pneumonia disease assessment using a slaughterhouse lung-scoring method. J Vet Med A Physiol Pathol Clin Med. 2007;54(2):70-75.
- Raymond BBA, Turnbull L, Jenkins C, et al. Mycoplasma hyopneumonia resides intracellularly within porcine epithelial cells. Scientific Reports. 2018; 8(1):17697.
- Sibila M, Pieters M, Molitor T, Maes D, Haesebrouck F, Segalés J. Current perspectives on the diagnosis and epidemiology of Mycoplasma hyopneumoniae infection. Vet J. 2009;181(3):221-31.