Signalment:
The laboratory has had a number of mice with head tilts in the past and is interested in determining the cause in order to treat or prevent further outbreaks of disease. This is an immune-compromised line that currently is not on antibiotic prophylaxis.
Gross Description:
Mouse 4: Head tilt to the right
Mice 1 4: White opaque material within the tympanic bullae
Mice 1, 2, 4: Swollen right forepaw (up to 0.6 CM in maximum dimension)
Mouse 3: Not foot lesions
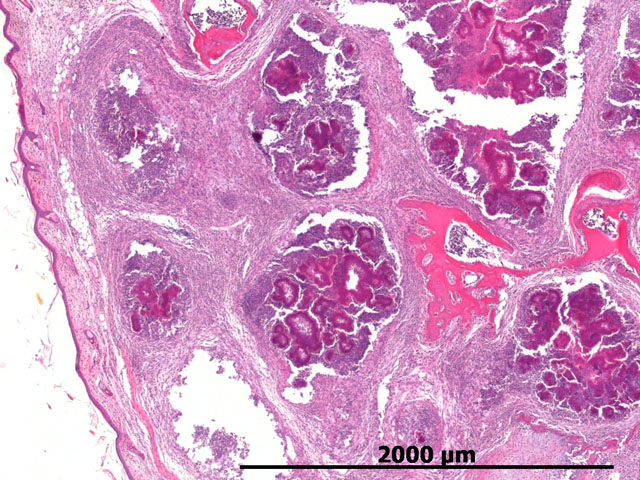
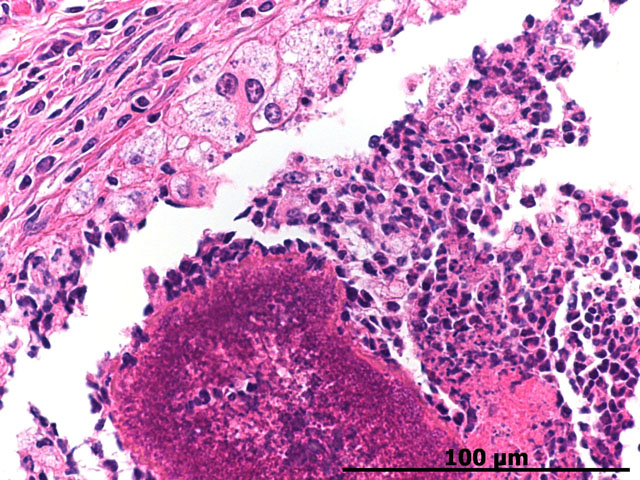
Histopathologic Description:
Morphologic Diagnosis:
Microbiology:
Mice 1 4: Nasal pharynx culture
Mice 1 3: Tympanic bulla culture
Mice 1, 2, 4: Forepaw cultured
Lab Results:
Microbiology Results
Initial
| Mouse | Site | Micro# | Bacteria | AMPIC | CEPHA | CHLOR | CIPRO | ERYTH | GENTA | LINCO | PENIC | SXT |
| 1 | Foot | MD717948 | S. aureus | R | S | S | S | R | S | |||
| 2 | Foot | MD717949 | S. aureus | R | S | S | S | R | S | |||
| 4 | Foot | MD717950 | S. aureus | R | S | S | S | R | S | |||
| P. mirabilis | S | S | S | R | S | S | ||||||
| 4 | Foot | MD717951 | S. aureus | R | R | S | S | S | R | S | ||
| 1-4 | NPX | MD717952 | P. mirabilis | Proteus mirabilis predominate in all the specimens from the Middle Ear, and 3 of 4 of the Nasal wash had it. | ||||||||
| 1-3 | Typanic Bulae | |||||||||||
Complete Antibiotic Culture and Sensitivity Profile: S = Susceptible; R = Resistant
| Antimicrobial | Staph. aureus | Proteus mirabilis |
| Amox/Clav AML | S | S |
| Ampicillin | R | S |
| Amikacin | S | S |
| Cefazolin | S | R |
| Ceftiofur | S | S |
| Cephalothin | S | S |
| Chloramphenicol | S | S |
| Ceftriaxone | S | S |
| Clindamycin | S | R |
| Doxycycline | S | R |
| Enrofloxacin | S | S |
| Gentamicin | S | S |
| Norfloxacin | S | S |
| Penicillin | R | S |
| Trimethroprim, Sulfamethoxazole | S | S |
Condition:
Contributor Comment:
Reported causative agents of botryomycosis include Staphylococcus aureus, S. hominus, S. xylosus, Pseudomonas aeruginosa, Proteus spp., Escherichia coli, Nocardia asteroides and Streptococcus intermedius, with a variety of other aerobic and anaerobic bacterial agents implicated.(3,9,11,14,15) Immunosuppression has been suggested as a factor in increased prevalence of botryomycosis.(11,16) As described above, the MyD88 knockout mouse is deficient in the innate immune response.
Splendore-Hoeppli staining refers to the deeply eosinophilic material that surrounds the -�-�grains of bacterial colonies contained centrally. The eosinophilic material is described as having a coronal appearance, which engulfs whitish purulent material associated with the colonies(3,14,15). Necrotic tissue has also been reported to be present.(3) While Splendore-Hoeppli bodies are predominately reported to be associated with botryomycosis, numerous reports of this phenomenon in the presence of parasitic (3) or fungal infections (4,15,16) are in the literature. The eosinophilic staining component of this histologic finding has been linked to cellular debris and antigen-antibody complexes.(3,11,15) This staining pattern has been referred to as Splendore-Hoeppli bodies, material, and phenomenon.
The lesions in the forepaw were subsequently determined to be secondary to toe-clip for identification. The colony was subsequently placed on Baytril and there were no further lesions.
JPC Diagnosis:
Conference Comment:
Botryomycosis usually manifests as a firm nodule that is ulcerated with a draining tract. The discharge often contains the previously described granules or spicules, thus suggesting botryomycosis. Histologically, this is a striking lesion with a marked pyogranulomatous response centered on bacterial colonies often encircled by Splendore-Hoeppli material. These lesions can be walled off by abundant fibrous connective tissue and can coalesce to form chains of granulomas within the subcutis and surrounding tissues.(6)
References:
2. Akira S: Toll-like receptors: lessons from knockout mice. Biochem Soc Trans 28:Part 5, 2000
3. Armed Forces Institute of Pathology: Wednesday Slide Conference, Conference 13, Case 1, AFIP #2812387, Conference Comments, 2006
4. Bersoff-Matcha Sj, Roper CC, Liapis H, and Little JR: Primary Pulmonary Botryomycosis: Case Report and Review. Clin Infec Dis 26:620624, 1998
5. EL van den Berk G, Noorduyn LA, van Ketel RJ, van Leeuwen J, Bemelman WA, and Prins JM: A fatal pseudo-tumour: disseminated basidiobolomycosis. BMC Infectious Diseases 6:140, 2006
6. Ginn PE, Mansell JEKL, Rakich PM: Skin and appendages. In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., pp. 691. Elsevier, Philadelphia, Pennsylvania, 2007
7. Goldstein DR, Tesar BM, Akira S, and Lakkis FG: Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest 111(10): 1571-1578, 2003
8. Kielian T, Phulwani NK, Esen N, Syed MM, Haney AC, McCastlain K, and Johnson J: MyD88- dependent signals are essential for the host immune response in experimental brain abscess. J Immunol 178:45284537, 2007
9. Machado CR, Schubach AO, Concei+�-�+�-�o-Silva F, Quintella LP, Louren+�-�o MCS, Carregal E, and do Valle ACF: Images in Clinical Dermatology. Dermatology 211:303-304, 2005
10. Rodig SJ and Dorfman DM: Splendore-Hoeppli phenomenon. Arch Pathol Lab Med 125:1515-1516, 2001
11. Schlossberg D, Pandey M, and Reddy R: The Splendore-Hoeppli phenomenon in hepatic botryomycosis. J Clin Pathol 51:399-400, 1998
12. Sugawara I, Yamada H, Mizuno S, Takeda K, and Akira S: Mycobacterial infection in MyD88- deficient mice. Microbiol Immunol 47(11):841-847, 2003
13. Tohno M, Skimazu T, Aso H, Kawai Y, Saito T, and Kitazawa H. Molecular Cloning and Functional Characterization of Porcine MyD88 Essential for TLR Signaling. Cell Mol Immunol 4(5):369-376, 2007
14. Weighardt H, Kaiser-Moore S, Vabulas RM, Kirschning CJ, Wagner H, and Holzmann B: Cutting edge: Myeloid differentiation factor 88 deficiency improves resistance against sepsis caused by polymicrobial infection. J Immnunol 169:28232827, 2002
15. Zaharopoulos P: Fine-needle aspiration cytologic diagnosis of lymphocutaneous sporotrichosis: A aase report. Diag Cytopathol 20(2):74-77, 1998
16. Zavasky D, Samowitz W, Loftus T, Segal H, and Carroll K: Gastrointestinal zygomycotic infection caused by basidiobolus ranarum: Case report and review. Clin Infec Dis 28(6):1244-8, 1999