WSC 22-23
Conference 6
CASE II:
Signalment:
0.5 years old, male, golden retriever (Canis familiaris)
History:
This dog was purchased through a breeder at 7 weeks of age. At around 4 months of age, the dog started having uncontrolled watery−mucoid diarrhea that was large volume and frequent. He was placed on Metronidazole and Proviable and needed to stay on the medication, as diarrhea would return when the drugs were not used. The dog was diagnosed with carpal flexure deformity at 3 months of age. Radiographs of the cervical spine and hips revealed mild hip dysplasia and shortened distal cervical vertebral bodies. The dog developed urinary accidents (normal volume) at home. On presentation to Iowa State University Teaching Hospital, the dog had lateral strabismus, vertical nystagmus, ataxia that was worse in hind limbs with normal reflexes and placement and hopping, head tremor, and rectal mucosa felt very irregular on rectal palpation. The owner did not allow many additional tests; only creatine kinase, which was mildly increased at 378, and blood smear which showed possible granules in lymphocytes. The dog’s condition deteriorated over a week and was euthanized.
Gross Pathology:
Small intestine: A small number (approximately 10) of 2−5 cm in length and 2 mm in diameter, white, elongate nematodes with tapered ends (roundworms) were present multifocally within the small intestinal lumen.
Laboratory Results:
Hepatic beta-hexosaminidase (Hex) An enzyme activity in the liver was elevated.
Microscopic Description:
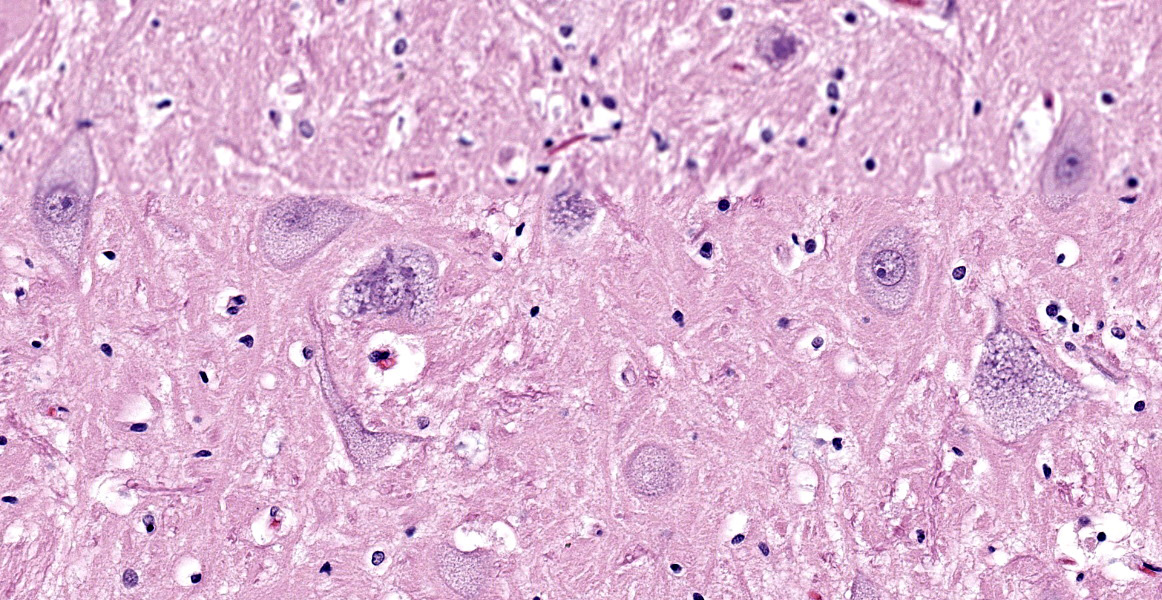
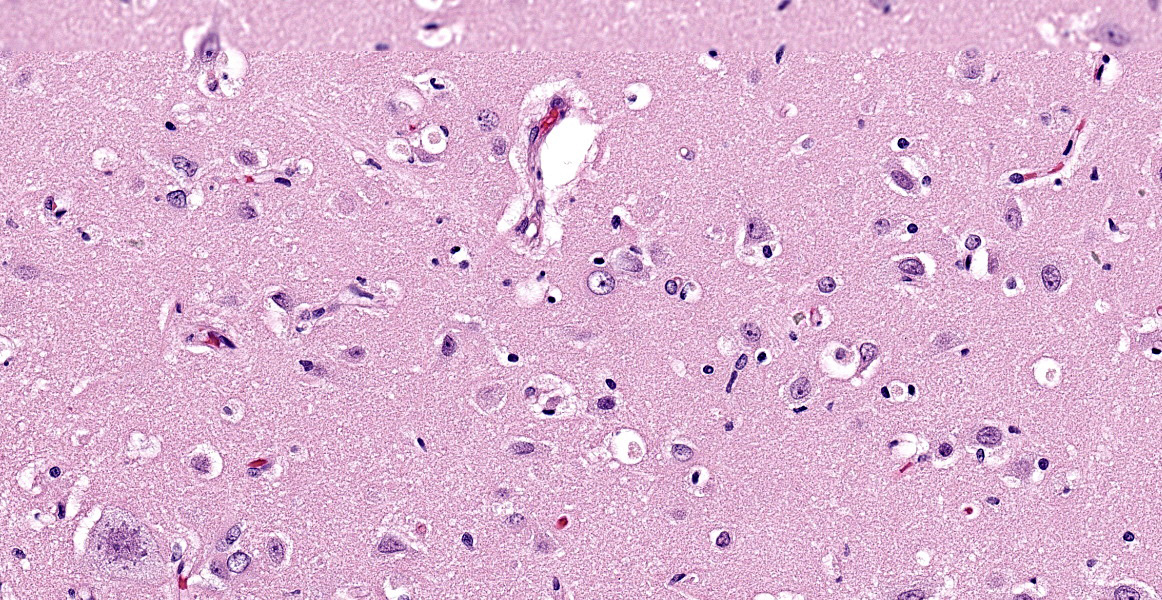
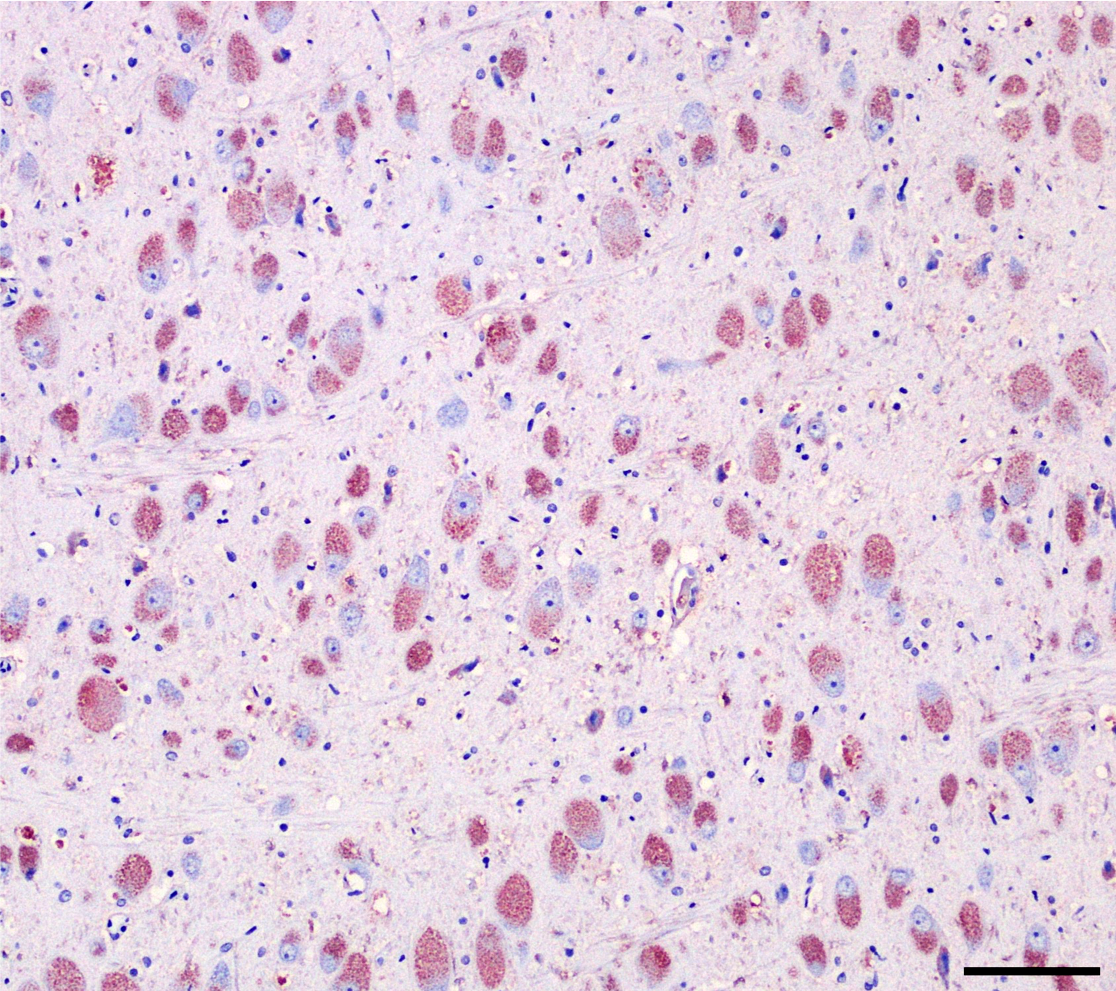
Brain (cerebral cortex at the level of internal capsule): Diffusely, neurons are moderate to markedly expanded by microvacuolated cytoplasm, which often peripheralizes the nucleus and occasionally extends into and expands proximal processes. Infrequently, neurons also contain larger vacuoles up to 8 microns in diameter. Microglia are subjectively increased in number. There is rare neuronal necrosis, and rare blood vessels are cuffed by few lymphocytes. White matter is mildly vacuolated with occasional spheroids and few vacuolated cells (small neuron or astrocyte morphology). The caudate nucleus is regionally hypercellular with vascular proliferation and gliosis − extant neurons are similarly vacuolated.
Other findings:
Spinal cord (Slides not submitted): All spinal cord segments are similarly affected. Diffusely, neurons are variably expanded by abundant finely vacuolated cytoplasm, which often peripheralizes the nucleus and Nissl substance. Multifocal neurons contain larger vacuoles, up to 8 microns in diameter, containing pale eosinophilic material. Some neurons contain both foamy microvacuolated cytoplasm and larger vacuoles. Glia appears unaffected. White matter and nerve rootlets contain scattered dilated and often empty axon sheaths. Meninges, blood vessels − no specific pathologic findings.
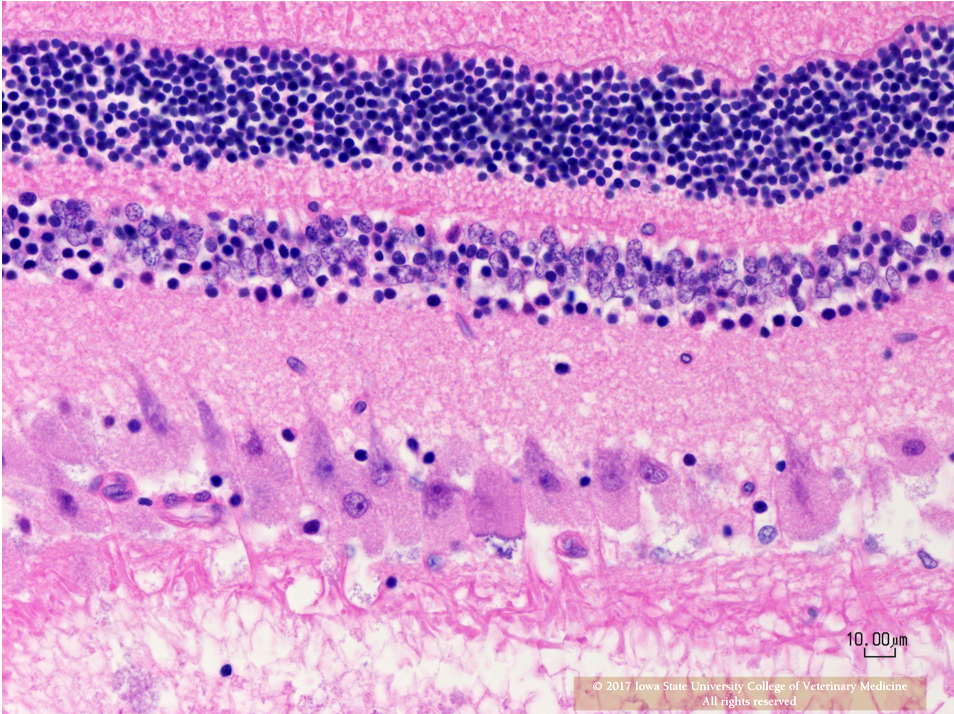
Eye (Slides not submitted): Retinal ganglion cells are similarly enlarged and vacuolated with margination of the nucleus.
Duodenum and colon (Slides not submitted): Myenteric and submucosal plexus neurons occasionally appear enlarged with microvacuolated clear to eosinophilic cytoplasm with marginated nuclei.
Urinary bladder (Slides not submitted): Ganglion cells within the bladder wall are similarly enlarged and vacuolated.
Mesenteric ganglion (Slides not submitted): Ganglion cells are markedly distended by similar microvaculated cytoplasm as described above.
Contributor’s Morphologic Diagnoses:
Cerebral cortex: Neuronal cytoplasmic vacuolation, diffuse, moderate to severe.
Spinal cord, mesenteric ganglia: Neuronal cytoplasmic vacuolation, diffuse, moderate to severe.
Retina: Retinal ganglion cell vacuolation, cytoplasmic, diffuse, moderate.
Contributor’s Comment:
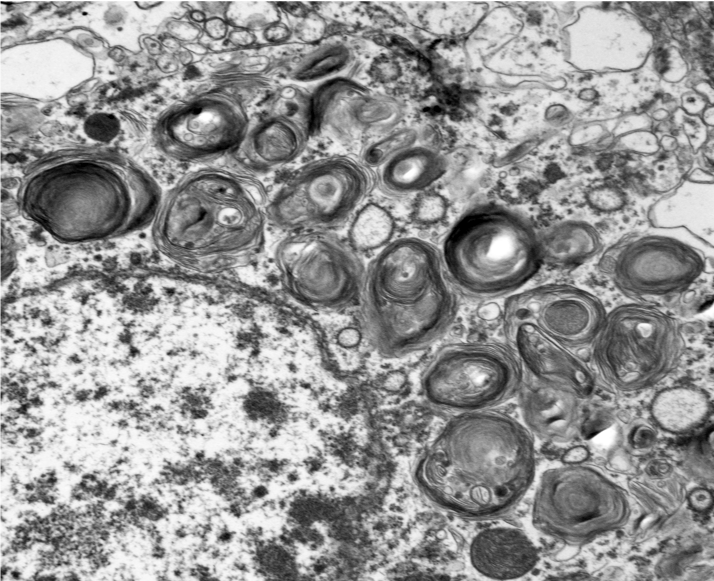
Microscopic changes in this dog were consistent with a lysosomal storage disease (LSD). Vacuolation was predominantly, if not solely, intraneuronal affecting the central, autonomic nervous systems, and retina. The vacuoles were positive for luxol fast blue (myelin stain) but negative for PAS stain, indicating the inclusions are composed predominately of lipid. Ultrastructural examination of the affected neurons revealed that the vacuoles contain concentric lamellated inclusions which are most consistent with the GM1 and GM2 gangliosidoses or sphingomyelinosis.
Sphingolipidoses are characterized by genetic defects in catabolism of glycosphingolipids which are normal components of cell membranes and extracellular matrix. In animals, based on protein defects, there are at least five important types, including GM1 gangliosidosis, GM2 gangliosidosis, sphingomyelinosis, galactocerebrosidosis, and glucocerebrosidosis (table 2-1)5-7. These protein defects lead to impaired function of lysosomal enzymes themselves or proteins assisting lysosomal enzymes in substrate processing. Ultrastructurally, the inclusions in GM1 and GM2 gangliosidoses and sphingomyelinosis reveal whorls and laminar arrangements, while the inclusions in galactocerebrosidosis and glucocerebrosidosis are characterized by long tortuous and twisted tubules.
GM1 gangliosidosis results from the deficiency of beta-galactosidase and shows accumulation of glycolipids and oligosaccharides in many cell types and tissues, including neurons, astrocytes, retinal cells, hepatocytes, Kupffer cells, and endothelial cells in different tissues. Canine GM1 gangliosidosis has an autosomal recessive pattern of inheritance.25 Different breeds reveal variable defects in the GLB1 (Galactosidase, beta 1) gene. In Portuguese Water Dogs, a G→A mutation in exon 2 results in an Arg→His amino acid substitution.22 In Shiba Inu dogs, the mutation is a deletion of C nucleotide 1668 in exon 15.23 Alaskan huskies reveal 19-bp duplication in exon 15 of GLB1. 12, 13 Due to similar genetic defects, clinical signs, and lesions, dogs are considered as a suitable animal model of human GM1 gangliosidosis.1, 8, 11, 22 In humans, GM1 gangliosidosis is divided into three types: type 1 (infantile), type 2 (late infantile/juvenile), and type 3 (adult). Except for the English springer spaniel, other canine breeds are comparable with the late infantile/juvenile form (Table 2-2).24 In this form, these dogs show progressive clinical nervous signs accompanied by possible skeletal abnormality and hepatosplenomegaly.
|
Table 2-1 Select sphingolipidoses |
|||||
|
Name |
Protein defect |
Ultrastructural feature of vacuole |
Histological features |
||
|
GM1 gangliosidosis |
beta-galactosidase |
Lamellar |
Vacuoles in neurons, astrocytes, endothelial cells, retinal cells, hepatocytes, and Kupffer cells |
||
|
GM2 gangliosidosis |
B variant (Tay-Sachs disease) |
beta-hexosaminidase (Hex) |
Alpha subunit of Hex A |
Lamellar |
Vacuoles in neurons and retinal ganglion cells |
|
O variant (Sandhoff disease) |
Beta subunit of Hex A and B |
||||
|
AB variant |
Activator protein |
||||
|
Sphingomyelinosis (Niemann- Pick disease) |
Sphingomyelinase |
Lamellar |
Vacuoles in macrophages and parenchymal cells in many tissues |
||
|
Cholesterol transporter |
|||||
|
Galactocerebrosidosis (galactosylceramide lipidosis, globoid cell leukodystrophy, Krabbe disease) |
Beta-galactocerebrosidase |
Tubular |
Vacuoles in oligodendrocytes, Schwann cells, macrophages, globoid cells, with demyelination |
||
|
Glucocerebrosidosis (glucosylceramidosis, Gaucher disease) |
Glucocerebrosidase |
Tubular |
Vacuoles in neurons, macrophages, Gaucher cells in the spleen, lymph nodes, liver, and bone marrow |
||
In this dog, elevated HexA activity ruled out B and O variants of GM2 gangliosidosis. Strong signal of cholera toxin subunit B in the cytoplasm of vacuolated neurons demonstrated the presences of GM1 ganglioside, potentially implicating GM1 gangliosidosis as the disease entity affecting the patient.9 However, GM1 ganglioside can also accumulate secondarily in other lysosomal storage diseases.19 Assay for enzyme deficiency or genetic analysis of the GLB1 gene is necessary for a definitive diagnosis of GM1 gangliosidosis. In this case, whole genome sequencing revealed a missense mutation in GLB1 resulting in a C→A substitution at exon 3. Additionally, beta galactosidase activity was significantly decreased in brain white matter, gray matter and liver. Activity levels of neuraminidase and protective protein/cathepsin A (PPCA) were normal. Taken together, these results are consistent with GM1 gangliosidosis, novel in the Golden Retriever breed, and most comparable to the late infantile/juvenile form in humans.
|
Table 2-2?Comparison of human and canine GM1 gangliosidosis |
||
|
Human |
Features |
Dog |
|
Type 1 (infantile) |
Dwarfism, facial distortion, bone deformities, hepatosplenomegaly, seizures, vision loss (early), startled response to sound |
English Springer Spaniel3 |
|
Type 2 (late infantile / juvenile) |
Bone deformities+/-, hepatosplenomegaly+/-, seizures, ataxia, spasticity, vision loss (late), startled response to sound |
German Shorthair Pointer9, Beagle/Mixed-breed14,15, Portuguese Water Dog2, Shiba Inu18, Alaskan husky13 |
|
Type 3 (adult) |
Ataxia, spasticity |
|
Contributing Institution:
Department of Veterinary Pathology
College of Veterinary Medicine
Iowa State University
https://vetmed.iastate.edu/vpath
JPC Diagnosis:
Cerebrum, neurons and glial cells: Vacuolation, cytoplasmic, diffuse, moderate, with mild gliosis.
JPC Comment:
Gangliosides have been studied extensively due to their role in gangliosidosis and the altered levels found in various neurodegenerative disorders such as Alzheimer’s disease.21 Gangliosides are a diverse group of molecules synthesized in the endoplasmic reticulum and Golgi apparatus. While found throughout the body, they are concentrated in the nervous system, residing in lipid rafts on the external plasma membrane surface of neuronal cells and synapses.21 Ganglioside prevalence changes with age; in humans, for instance, GM1 concentration increases until adulthood and gradually decreases in old age.21 While elevated concentration of gangliosides can cause severe disease, in certain conditions, gangliosides can have neurotrophic or neuroprotective effects.21 Human trials, for instance, have demonstrated that exogenous GM1 helped alleviate neurotoxicity associated with chemotherapy.21
In addition to various breeds of dogs, gangliosidoses have been reported in cats, sheep, cattle, pigs American black bears, and emus.4,15 In sheep, GM1 ganlgiosidosis has been reported in the Suffolk (type 1) and Romney (types 1, 2, and 3) breeds; in cats, it has been documented in Siamese, Korat, and mixed breed cats.18,20
Muthupalani et al. described seven young free-ranging American black bears in New England with poor body condition and variable neurologic symptoms including tremors, ataxia, and hypermetria. The animals had few vacuolated mononuclear cells and neutrophils on blood smear and cerebral atrophy with enlarged ventricles and prominent sulci on necropsy. 15 Eosinophilic vacuoles were present in neurons, retinal ganglion cells, renal proximal tubular epithelium, chondrocytes, and hepatocytes; the vacuoles in the neurons and retina were blue on Luxol fast blue staining. 15 Enzymatic testing revealed drastically decreased beta galactosidase activity compared to the control, and gene sequencing identified a missense mutation Y348H on exon 10 in the GLB1 gene. 15 These findings were consistent with GM1-gangliosidosis. 15
Bermudez et al described two adolescent emu hatchmates with progressive neurologic signs had similar vacuoles reported in neurons of the cerebrum, brainstem, cerebellum, spinal and autonomic ganglia, and retina. 4 GM1 and GM3 levels were up to 25 times higher than controls, and decreased beta-galactosidase activity; these findings were all consistent with gangliosidosis.4 This was the first documented report of a neuronal storage disease in avian species outside of Lafora body neuropathy.
References:
- Ahern-RindellAJ, KretzKA, O’BrienJS. Comparison of the canine and human acid beta-galactosidase gene. Am J Med Genet. 1996 May 17 [cited 2018 May 27];63:340-345.
- AlroyJ, OrgadU, DeGasperiR, et al. Canine GM1-gangliosidosis. A clinical, morphologic, histochemical, and biochemical comparison of two different models. Am J Pathol. 1992;140:675-689.
- AlroyJ, OrgadU, UcciAA, et al. Neurovisceral and skeletal GM1-gangliosidosis in dogs with beta-galactosidase deficiency. Science. 1985 Aug 2 [cited 2018 May 27];229:470-472.
- Bermudez AJ, Johnson GC, Vanier MT, et al. Gangliosidosis in Emus (Dromaius novaehollandiae). Avian Diseases. 1995; 39:292-303.
- CantileC, Youssef S. Nervous System. In: Maxi MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 1. 6th Philadelphia, PA: Elsevier Saunders. 2016:287-288.
- Cheville NF. Metabolic and Storage Diseases. In: Ultrastructural Pathology The Comparative Cellular Basis of Disease. Wiley-Blackwell; 2009:856-861.
- Lysosomal disorders of the nervous system. In: Neurobiology of Disease. Elsevier; 2011:1-9.
- HubertJJ, O ‘brienJS. Dog and human acid beta-D-galactosidases are structurally similar. Biochem J. 1983;213:473-478.
- IwamasaT, OhshitaT, NashiroK, IwanagaM. Demonstration of GM1-ganglioside in nervous system in generalized GM1-gangliosidosis using cholera toxin B subunit. Acta Neuropathol. 1987 [cited 2018 May 27];73:357-360.
- Karbe E, Schiefer B. Familial Amaurotic Idiocy in Male German Shorthair Pointers. Pathol Vet. 1967; 4(3):223-32. [cited 2018 May 27].
- Kaye EM, Alroy J, Raghavan SS, et al. Dysmyelinogenesis in animal model of GM1 gangliosidosis. Pediatr Neurol. 1992 [cited 2018 May 27];8:255-261.
- KreutzerR, KreutzerM, PröpstingMJ, et al. Insights into post-translational processing of β-galactosidase in an animal model resembling late infantile human GM1-gangliosidosis. J Cell Mol Med. 2008;12:1661-1671.
- Kreutzer R, Leeb T, Müller G, Moritz A, Baumgärtner W. A duplication in the canine β-galactosidase gene GLB1 causes exon skipping and GM1-gangliosidosis in Alaskan huskies. Genetics. 2005;170:1857-1861.
- Lldinger SA, Oritz AM, Urbriggen AZ, Irchhof NK, Ewell AS, Aumga WB. GM 1 -gangliosidosis in Alaskan Huskies: Clinical and Pathologic Findings. Vet Pathol. 2001; 38(3); 281-290.
- Muthupalani S, Torres PA, Wang BC, et al. GM1-gangliosidosis in American black bears: Clinical, pathological biochemical, and molecular genetic characterization.Mol Genet Metab. 2014; 111(4): 513-521.
- Read DH, Harrington DD, Keenana TW, Hinsman EJ. Neuronal-visceral GM1 gangliosidosis in a dog with beta-galactosidase deficiency. Science. 1976 Oct 22 [cited 2018 May 27];194:442-445.
- RodriguezM, O’BrienJS, GarrettRS, PowellHC. Canine GM1 gangliosidosis. An ultrastructural and biochemical study. J Neuropathol Exp Neurol. 1982 Nov [cited 2018 May 27];41:618-629.
- Ryder SJ, Simmons MM. A lysosomal storage disease of Romney sheep that resembles human type 3 GM1 gangliosidosis. Acta Neuropathol. 2001; 101:225-228. Mol Genet Metab. 2014; 111:513-521.
- Satoh H, Yamato O, Asano T, Yamasaki M, Maede Y. Increased concentration of G M1 -ganglioside in cerebrospinal fluid in dogs with GM1- and GM2-gangliosidoses and its clinical application for diagnosis. J Vet Diagn Invest. 2004;16:223-226.
- Uddin MM, Hossain MA, Rahman MM, et a. Identification of Bagladeshi Cats with GM1 Gangliosidosis caused by the c.1448>C Mutation of the Feline GLB1 Gene: Case Study. J Vet Med Sci. 2013; 75(3):395-397.
- Vasques JF, de Jesus Goncalves RG, da Silva-Junior AJ, Martins RS, Gubert F, Mendez-Otero R. Gangliosides in nervous system development, regeneration, and pathologies. Neural Regeneration Research. 2023: 18(1): 81-86.
- WangZH, ZengB, ShibuyaH, et al. Isolation and characterization of the normal canine beta-galactosidase gene and its mutation in a dog model of GM1-gangliosidosis. J Inherit Metab Dis. 2000;23:593-606.
- Yamato O, Endoh D, Kobayashi A, et al. A novel mutation in the gene for canine acid beta-galactosidase that causes GM1-gangliosidosis in Shiba dogs. J Inherit Metab Dis. 2002 Oct [cited 2018 May 27];25:525-526.
- YamatoO, MasuokaY, YonemuraM, et al. Clinical and clinico-pathologic characteristics of Shiba dogs with a deficiency of lysosomal acid beta-galactosidase: a canine model of human GM1 gangliosidosis. J Vet Med Sci. 2003;65:213-217.
- Yamato O, Ochiai K, Masuoka Y, et al. GM1 gangliosidosis in shiba dogs. Vet Rec. 2000;146:493-496.