Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 17
30 January, 2019
CASE II: L14 10949 (JPC 4066258)
Signalment: Two 2.5 week old male intact, Spots breed, Suidae, domestic pigs (litter mates).
History: Healthy litter of pigs - 2 1/2 weeks old. Owner noticed rapid breathing in one piglet, and it was given penicillin G and iron dextran injections. The pig died immediately. A second pig in the litter developed similar breathing problems and died after being given Florfenicol. A third piglet with similar signs received Florfenicol, Flunixin and Dexamethasone with some improvement noted. All three piglets developed signs in the span of a few hours. The two dead piglets were submitted for necropsy.
Gross Pathology: Gross alterations were similar in both pigs. The pericardial sac contained approximately 5 ml of serosanguinous fluid. The epicardium had multifocal to coalescing white areas that on cut surface extended into the myocardium. The lungs were diffusely wet, non-collapsing and mottled light red to light purple, with prominent interlobular septa. Approximately 30 ml of serosanguinous fluid was present in the peritoneal cavity, and scattered delicate fibrin strands covered the serosal surface of the intestines. Diffuse accentuated lobular pattern was evident throughout the liver, which appeared to be more friable than usual.
Laboratory results: Bacteriology: Lung - No bacteria isolated
Parasitology: Colon contents - No parasite eggs seen
Virology:
Lung and spleen - Negative for Porcine Reproductive and Respiratory Syndrome virus (PRRSV) by fluorescent antibody
Lung, intestine, and spleen - Negative for Porcine Circovirus-2 (PCV2) by fluorescent antibody
Intestine - Negative for Transmissible Gastroenteritis (TGE) virus and Porcine Rotavirus by fluorescent antibody assay, and for enteric viruses by electron microscopy
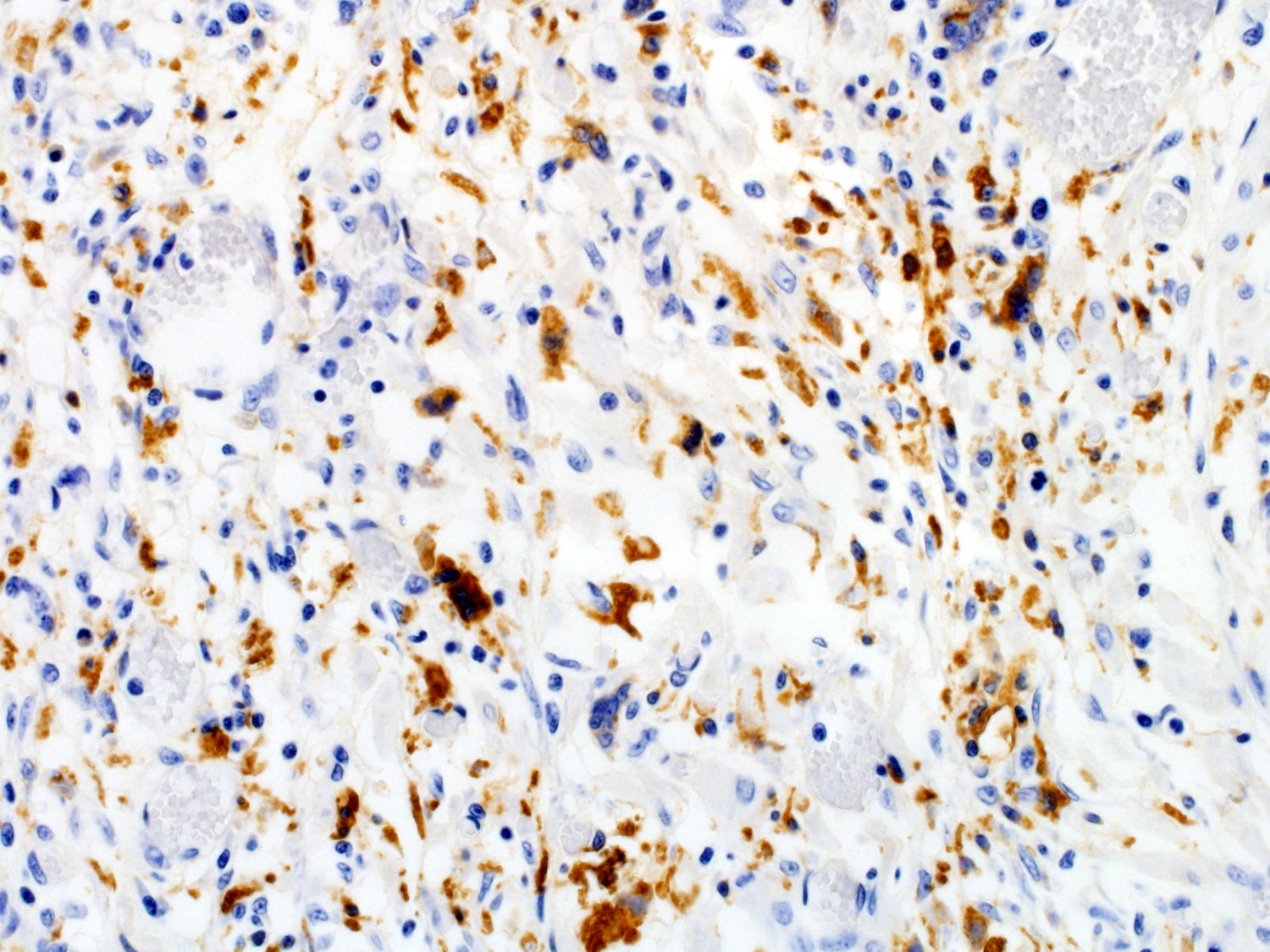
Heart - Negative for Encephalomyocarditis virus (EMCV) by PCR Immunohistochemistry (performed at the University of Pennsylvania):
Heart - Immunopositive for PCV2 antigen in cardiomyocytes, endothelial cells, and infiltrating macrophages
Microscopic Description:
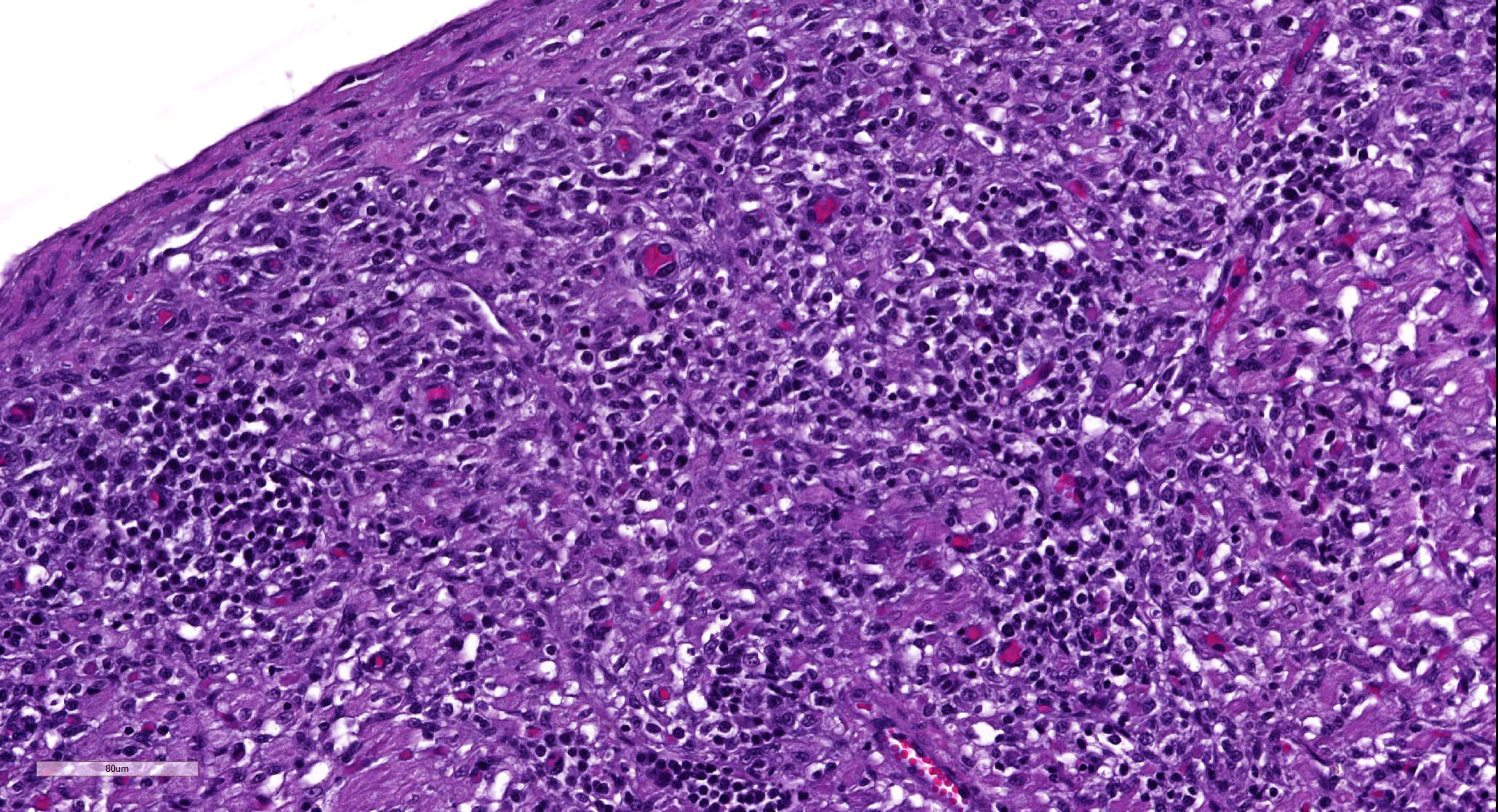
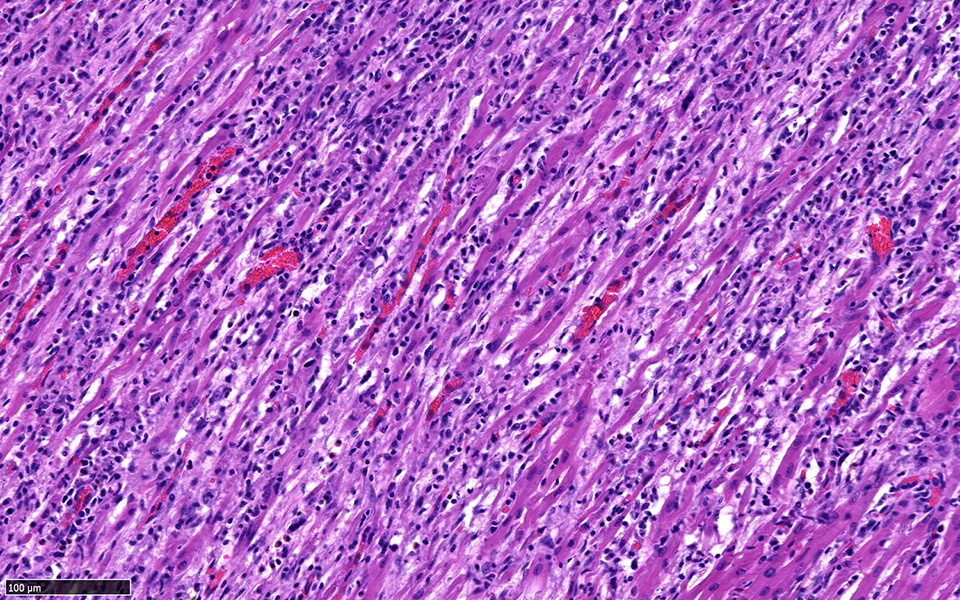
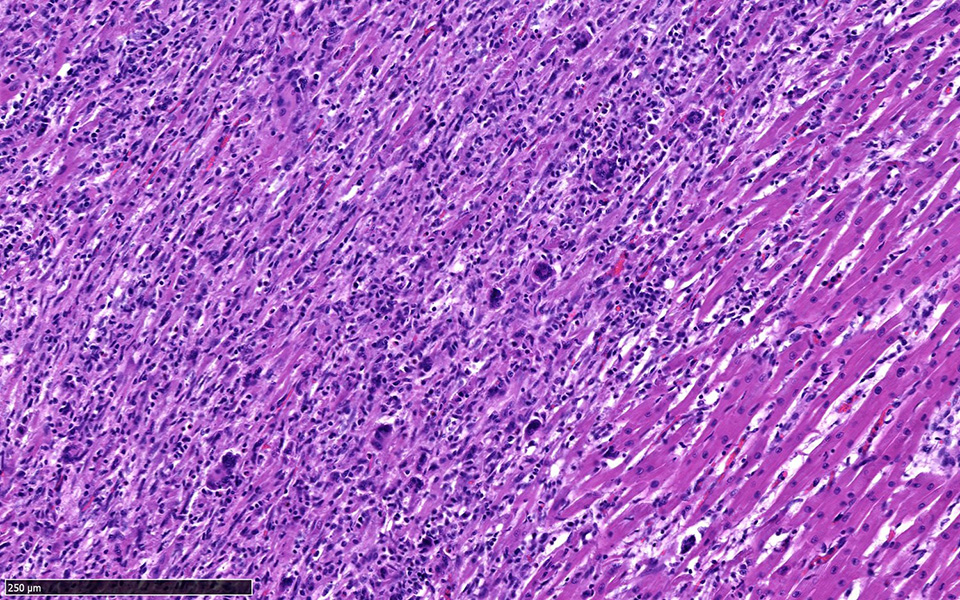
Heart: Approximately fifty percent of the myocardium of the right and left free ventricular walls as well as of the interventricular septum is multifocally disrupted and replaced by inflammatory infiltrates of lymphocytes and plasma cells, fewer histiocytes and eosinophils, and sporadic multinucleated giant cells. Inflammatory cells also multifocally infiltrate the epicardium and endocardium. Cardiomyocytes are frequently characterized by loss of cross-striations, sarcoplasmic vacuolation and fragmentation, and nuclear swelling or pyknosis (myocardial degeneration and necrosis). Occasional cardiomyocyte abortive regeneration is suspected based on the presence of some giant cells with mildly basophilic cytoplasm and clusters of large euchromatic nuclei. Lost myocardium is often replaced by fibrous connective tissue.
Additional not shown histologic findings in both pigs consisted of centrilobular hepatocellular degeneration and necrosis, erythroid hyperplasia in the bone marrow, and extramedullary hematopoiesis in multiple tissues suggestive of anemia, as well as subacute pulmonary edema and congestion consistent with subclinical cardiac insufficiency.
Contributor’s Morphologic Diagnoses:
Heart: Myocarditis, lymphoplasmacytic and histiocytic, multifocal to coalescing, chronic, severe, with fibrosis
Contributor’s Comment: Porcine circovirus type 2 (PCV2) is a small, non-enveloped DNA virus. It was first described as the causative agent of postweaning multisystemic wasting syndrome (PMWS), a disease with a wide variety of clinical signs and lesions in multiple organ systems. Classically, PMWS is characterized by dyspnea, diarrhea, pallor, and jaundice with severe lymphoid depletion in pigs between 5 and 8 weeks of age. In addition to causing PMWS, PCV2-infection has been associated with an array of conditions, including respiratory and enteric diseases, porcine dermatitis and nephropathy syndrome (PDNS), and reproductive failure.7
The submitted case is an example of PCV2-associated myocarditis. PCV-associated myocarditis is most commonly seen in aborted, mummified, and weak-born fetuses that were infected during gestation.6,7,11 However, chronic myocarditis has also rarely been reported in piglets infected at 1-3 days of age,2,3,4,8 and we believe that this is what occurred in the current case. In previous reports, cardiac lesions tended to be more severe in animals co-infected with porcine parvovirus.2,8 We did not test for parvovirus; we can therefore not rule it out as a contributing factor to disease in our case. Additional causes of myocarditis in swine including bacteria (e.g. Streptococcus suis and other bacteria associated with septicemia) and other viruses (e.g. PRRSV and EMCV) were ruled out by negative test results.5
In the current case, tissues routinely tested for the presence of PCV2 antigen (multiple lymph nodes, spleen, and lung) yielded negative results by immunofluorescence. These tissues also had no inflammatory lesions on histology. The heart, however, was positive by immunohistochemistry for PCV2 antigen, and this correlated well with the presence of the cardiac lesions. These findings illustrate the importance of being able to recognize potential PCV2-associated lesions in order to then submitting the affected tissues to PCV2 antigen testing. Similarly, only affected tissues should be used for detection of PCV2 by PCR, since the mere presence of PCV2 DNA in the absence of lesions is not sufficient for a definitive disease diagnosis.7
Contributing Institution:
Louisiana Animal Disease Diagnostic Laboratory
http://www1.vetmed.lsu.edu/laddl/index.html
JPC Diagnosis: Heart: Pancarditis, lymphoplasmacytic, histiocytic, and necrotizing, multifocal to coalescing, severe with multinucleated giant cells.
JPC Comment: The family Circoviridae is a rapidly expanding family of small, non-enveloped, circular, single-stranded, DNA viruses. Although chicken anemia agent, an early addition, has now been booted to the Anelloviridae, circoviruses have been identified in swine, a wide variety of bird species (including psittacine beak and feather disease), dogs, bats, mink, pandas, hermit crabs, and insects.12
A number of interesting syndromes have been ascribed to PCV-associated diseases (PCVAD). Previous WSC submissions falling into this category include a previous case of PCV-associated myocarditis (which also has an excellent comment on PCVAD and post-weaning multisystemic wasting syndrome (PMWS) – WSC 2016, Conf 8, Case 2), cerebellar vasculitis and necrosis (WSC 2014, Conf 21, Case 4), granulomatous lymphadenitis and hepatitis (WSC 2013 Conf 25 Case1) and tubulointerstitial nephritis (WSC 2011, Conference 7, Case 3.
Lymphohistiocytic myocarditis is considered a hallmark lesion in the prenatal infection of pigs with porcine circovirus-2 (PCV-2). Experimental infection of swine fetuses at day 52 is associated with myocardial viral tropism versus later infection (after day 92) which is generally associated with lymphoid tropism. 1 In affected fetuses, myofiber degeneration, necrosis, and loss and replacement by collagen and mineral, as well as inflammatory changes similar to those seen in this case and considered hallmark signs. Classic PCV-2-associated botryoid intranuclear inclusions (not seen in this section) may also be present. Cardiomegaly (predominantly right sided and associated with pericardial effusion) has been reported in piglets aged 4-7 weeks in heart failure.7
Vasculitis is also a well-described lesion in association with PCV-2 infection. Porcine dermatitis and nephritis syndrome is characterized by a necrotizing and neutrophilic vasculitis of arterioles and capillaries of the skin and kidney (to include glomerular capillaries). Fibrinoid necrosis of septal capillaries within the lung and resultant focal alveolar hemorrhage and edema has been widely reported in the US and Europe7. Finally, cases of myocarditis in piglets are often associated with lymphohistiocytic coronary arteritis and periarteritis.7,8
Within the last several years, a novel swine circovirus (PCV-3) has been identified by independent groups that has been identified with cardiac and multisystemic infection.10,11 In one study, both individual swine and a 2% of a thousand-animal herd displayed common lesions of lymphohistiocytic myocarditis and cardiac arteriolitis. Metagenomic testing identified a new circovirus showing 55% and 33% with bat circovirus and porcine circoviruses.11 A separate group in Kansas identified a similar virus, also identified as PCV-3 in sows that died acutely with lesions consistent with porcine dermatitis and nephritis syndrome.10
The moderator reviewed circoviruses in general, in pigs, dogs, and bears among other species, and a differential diagnosis for the lesion in this suckling piglet, including porcine circovirus-3, parvovirus, and aphthovirus was discussed.
References:
- Cushing T, Steffen D, Duhamel GE. Pathology in Practice. JAVMA 2013; 242(3):317-319.
- Ellis J, Krakowka S, Lairmore M, et al. Reproduction of lesions of postweaning multisystemic wasting syndrome in gnotobiotic piglets. Journal of Veterinary Diagnostic Investigation 1999;11:3-14.
- Hirai T, Nunoya T, Ihara T, Kusanagi K, Kato T, Shibuya K. Acute hepatitis in a piglet experimentally inoculated with tissue homogenates from pigs with postweaning multisystemic wasting syndrome. Journal of Veterinary Medical Science 2003;65:1041-1045
- Kennedy S, Moffet D, McNeilly F, et al. Reproduction of lesions of postweaning multisystemic wasting syndrome by infection of conventional pigs with porcine circovirus 2 alone or in combination with porcine parvovirus. Journal of Comparative Pathology 2000;122:9-24.
- Loynachan AT. 2012. Cardiovascular and Hematopoietic Systems – Diseases of the Myocardium. In Zimmerman JJ, Karriker LA, Ramirez A, et al. (Eds.), Diseases of Swine. Ames, IA: Wiley-Blackwell. p. 192-193.
- Mikami O, Nakajima H, Kawashima K, et al. Nonsuppurative myocarditis caused by porcine circovirus type 2 in a weak born piglet. Journal of Veterinary Medical Science 2005;67:735-738.
- Opriessnig T, Langohr I. Current state of knowledge on porcine circovirus type 2-associated lesions. Veterinary Pathology 2013;50:23-38.
- Opriessnig T, Janke B, Halbur P. Cardiovascular lesions in pigs naturally or experimentally infected with porcine circovirus type 2. Journal of Comparative Pathology 2006;134:105-110.
- Palinski R, Pineyro P, Shang P, Yuan F, Guo R, Fang Y, Byers E, Hause BM. A novel porcine circovirus distantly related to known circoviruses is associated with porcine dermatitis and nephropathy syndrome and reproductive failure. J Virol 2017 91(10:e01879-16.
- Phan TG, Giannitti F, Rossow S, Marhaler D, Knutson T, Li L, Deng X, Resende T, Vannuci F, Delwart. Detection of a novel circovirus PCV-3 in pigs with cardiac and multi-systemic inflammation. Virol J 2016; 13:184.
- Sanchez R, Nauwynck H, McNeilly F, et al. Porcine circovirus 2 infection in swine foetuses inoculated at different stages of gestation. Veterinary Microbiology 2001;83:169-176.
- Sheykhi A, Sheiki N, Charkhkar S, Brujeni GN. Detection and characterization of circovirus in canary flocks. Avian Dis 2018; 62:137-142.